Abstract
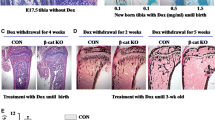
Mature osteoblasts have three fates: as osteocytes, quiescent lining cells, or osteoblasts that undergo apoptosis. However, whether intermittent parathyroid hormone (PTH) can modulate the fate of mature osteoblasts in vivo is uncertain. We performed a lineage-tracing study using an inducible gene system. Dmp1-CreERt2 mice were crossed with Rosa26R reporter mice to obtain targeted mature osteoblasts and their descendants, lining cells or osteocytes, which were detected using X-gal staining. Rosa26R:Dmp1-CreERt2(+) mice were injected with 0.25 mg 4-OH-tamoxifen (4-OHTam) on postnatal days 5, 7, 9, 16, and 23. In a previous study, at 22 days after the last 4-OHTam, most LacZ+ cells on the periosteal surface were inactive lining cells. On day 25 (D25), the mice were challenged with an injection of human PTH (1–34, 80 μg/kg) or vehicle daily for 10 (D36) or 20 days (D46). We evaluated the number and thickness of LacZ+ osteoblast descendants in the calvaria and tibia. In the vehicle group, the number and thickness of LacZ+ osteoblast descendants at both D36 and D46 significantly decreased compared to D25, which was attenuated in the PTH group. In line with these results, PTH inhibited the decrease in the number of LacZ+/osteocalcin-positive cells compared to vehicle at both D36 and D46. As well, the serum levels of sclerostin decreased, as did the protein expression of sclerostin in the cortical bone. These results suggest that intermittent PTH treatment can increase the number of periosteal osteoblasts by preventing mature osteoblasts from transforming into lining cells in vivo.





Similar content being viewed by others
References
Marie PJ (2008) Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys 473:98–105
Kartsogiannis V, Ng KW (2004) Cell lines and primary cell cultures in the study of bone cell biology. Mol Cell Endocrinol 228:79–102
Aubin JE, Triffitt JT (2002) Mesenchymal stem cells and osteoblast differentiation. In: Bilezikian JP, Raisz LG (eds) Principles of bone biology, 1st edn. Academic Press, San Diego, pp 59–81
Maes C, Kobayashi T, Kronenberg HM (2007) A novel transgenic mouse model to study the osteoblast lineage in vivo. Ann N Y Acad Sci 1116:149–164
Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM (2010) Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell 19:329–344
Kim SW, Pajevic PD, Selig M, Barry KJ, Yang JY, Shin CS, Baek WY, Kim JE, Kronenberg HM (2012) Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J Bone Miner Res 27:2075–2084
Franz-Odendaal TA, Hall BK, Witten PE (2006) Buried alive: how osteoblasts become osteocytes. Dev Dyn 235:176–190
Divieti Pajevic P (2013) Recent progress in osteocyte research. Endocrinol Metab (Seoul) 28:255–261
Jilka RL, O’Brien CA, Ali AA, Roberson PK, Weinstein RS, Manolagas SC (2009) Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts. Bone (NY) 44:275–286
Powell WF Jr, Barry KJ, Tulum I, Kobayashi T, Harris SE, Bringhurst FR, Pajevic PD (2011) Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J Endocrinol 209:21–32
Soriano P (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21:70–71
Chung UI, Lanske B, Lee K, Li E, Kronenberg H (1998) The parathyroid hormone/parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc Natl Acad Sci USA 95:13030–13035
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Jilka RL (2007) Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone (NY) 40:1434–1446
Kalajzic I, Braut A, Guo D, Jiang X, Kronenberg MS, Mina M, Harris MA, Harris SE, Rowe DW (2004) Dentin matrix protein 1 expression during osteoblastic differentiation, generation of an osteocyte GFP-transgene. Bone (NY) 35:74–82
Gluhak-Heinrich J, Ye L, Bonewald LF, Feng JQ, MacDougall M, Harris SE, Pavlin D (2003) Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo. J Bone Miner Res 18:807–817
Moester MJ, Papapoulos SE, Lowik CW, van Bezooijen RL (2010) Sclerostin: current knowledge and future perspectives. Calcif Tissue Int 87:99–107
Watanabe T, Tamamura Y, Hoshino A, Makino Y, Kamioka H, Amagasa T, Yamaguchi A, Iimura T (2012) Increasing participation of sclerostin in postnatal bone development, revealed by three-dimensional immunofluorescence morphometry. Bone (NY) 51:447–458
Hikita A, Iimura T, Oshima Y, Saitou T, Yamamoto S, Imamura T (2015) Analyses of bone modeling and remodeling using in vitro reconstitution system with two-photon microscopy. Bone (NY) 76:5–17
Acknowledgments
We thank Dr. Hank Kronenberg for kindly providing us with Dmp1-CreERt2 and Rosa26R mice. This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. 2010-0003236).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors state that they have no conflict of interest.
Additional information
M. Jang and J.Y. Lee contributed equally to this work.
About this article
Cite this article
Jang, MG., Lee, J.Y., Yang, JY. et al. Intermittent PTH treatment can delay the transformation of mature osteoblasts into lining cells on the periosteal surfaces. J Bone Miner Metab 34, 532–539 (2016). https://doi.org/10.1007/s00774-015-0707-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-015-0707-x