Abstract
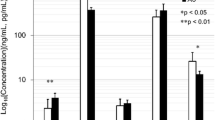
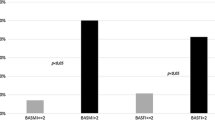
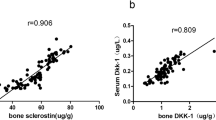
Sclerostin and dickkopf-1(DKK1) are Wnt/β-catenin signal antagonists that play an important role in bone formation. Ossification of the posterior longitudinal ligament (OPLL) of the spine is characterized by pathological ectopic ossification of the posterior longitudinal ligament and ankylosing spinal hyperostosis. The aims of this study were to evaluate serum sclerostin and DKK1 levels in persons with OPLL and to identify its relationship with bone metabolism and bone mass in persons with OPLL. This was a case–control study, and 78 patients with OPLL were compared with 39 age- and sex-matched volunteers without OPLL. We analyzed the relationship with calciotropic hormones, bone turnover markers, OPLL localization, number of ossified vertebrae, and bone mineral density of total hip (TH-BMD). Serum sclerostin levels in men with OPLL were significantly higher than in men in the control group (control group: mean = 45.3 pmol/L; OPLL group: mean = 75.7 pmol/L; P = 0.002). Age and sclerostin levels were positively correlated in men with OPLL (r = 0.43; P = 0.002). Serum sclerostin levels in men with OPLL had a positive correlation with TH-BMD Z-score (r = 0.511; P = 0.011, n = 30). There was a strong negative correlation between serum sclerostin levels and serum DKK1 levels in men with OPLL (r = −0.506; P < 0.001). Bone and mineral metabolism in OPLL differs between men and women. In men with OPLL, systemic secretion of sclerostin increases with advancing age and with higher bone mass. These two Wnt/β-catenin signal antagonists have the opposite effect in persons with OPLL, and higher serum sclerostin levels are counterbalanced by underproduction of DKK1.



Similar content being viewed by others
References
Tsuyama N (1984) Ossification of the posterior longitudinal ligament of the spine. Clin Orthop Relat Res 184:71–84
Resnick D, Guerra J Jr, Robinson CA, Vint VC (1978) Association of diffuse idiopathic skeletal hyperostosis (DISH) and calcification and ossification of the posterior longitudinal ligament. Am J Roentgenol 131:1049–1053
Taguchi T (2006) Etiology and Pathogenesis. In: Yonenobu K, Nakamura K, Toyama Y (eds) OPLL; Ossification of the posterior longitudinal ligament, 2nd edn. Springer Japan, Tokyo, pp 33–35
Kawaguchi H, Akune T, Ogata N, Seichi A, Takeshita K, Nakamura K (2006) Contribution of metabolic conditions to ossification of the posterior longitudinal ligament of spine. In: Yonenobu K, Nakamura K, Toyama Y (eds) OPLL; Ossification of the posterior longitudinal ligament, 2nd edn. Springer Japan, Tokyo, pp 37–40
Kawaguchi H, Kurokawa T, Hoshino Y, Kawahara H, Ogata E, Matsumoto T (1992) Immunohistochemical demonstration of bone morphogenetic protein-2 and transforming growth factor-beta in the ossification of the posterior longitudinal ligament of the cervical spine. Spine 17:S33–S36
Akune T, Ogata N, Seichi A, Ohnishi I, Nakamura K, Kawaguchi H (2001) Insulin secretory response is positively associated with the extent of ossification of the posterior longitudinal ligament of the spine. J Bone Joint Surg Am 83-A:1537–1544
Nakajima M, Takahashi A, Tsuji T, Karasugi T, Baba H et al (2014) A genome-wide association study identifies susceptibility loci for ossification of the posterior longitudinal ligament of the spine. Nat Genet 46:1012–1016
Matsui H, Yudoh K, Tsuji H (1996) Significance of serum levels of type I procollagen peptide and intact osteocalcin and bone mineral density in patients with ossification of the posterior longitudinal ligaments. Calcif Tissue Int 59:397–400
Hirai N, Ikata T, Murase M, Morita T, Katoh S (1995) Bone mineral density of the lumbar spine in patients with ossification of the posterior longitudinal ligament of the cervical spine. J Spinal Disord 8:337–341
Yamauchi T, Taketomi E, Matsunaga S, Sakou T (1999) Bone mineral density in patients with ossification of the posterior longitudinal ligament in the cervical spine. J Bone Miner Metab 17:296–300
Tahara M, Aiba A, Yamazaki M, Ikeda Y, Goto S, Moriya H, Okawa A (2005) The extent of ossification of posterior longitudinal ligament of the spine associated with nucleotide pyrophosphatase gene and leptin receptor gene polymorphisms. Spine 30:877–881
Sohn S, Chung CK (2013) Increased bone mineral density and decreased prevalence of osteoporosis in cervical ossification of the posterior longitudinal ligament: a case-control study. Calcif Tissue Int 92:28–34
Yoshimura N, Nagata K, Muraki S, Oka H, Yoshida M, Enyo Y, Kagotani R, Hashizume H, Yamada H, Ishimoto Y, Teraguchi M, Tanaka S, Kawaguchi H, Toyama Y, Nakamura K, Akune T (2014) Prevalence and progression of radiographic ossification of the posterior longitudinal ligament and associated factors in the Japanese population: a 3-year follow-up of the ROAD study. Osteoporos Int 25:1089–1098
van Buchem FS, Hadders HN, Ubbens R (1955) An uncommon familial systemic disease of the skeleton: hyperostosis corticalis generalisata familiaris. Acta Radiol 44:109–120
Balemans W, Ebeling M, Patel N, Van Hul E, Olson P et al (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10:537–543
Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J (2001) Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 68:577–589
Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Löwik CW, Reeve J (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 19:1842–1844
van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Löwik CW (2004) Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 15:805–814
Piters E, Boudin E, Van Hul W (2008) Wnt signaling: a win for bone. Arch Biochem Biophys 473:112–116
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192
Canalis E (2013) Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat Rev Endocrinol 9:575–583
Viapiana O, Fracassi E, Troplini S, Idolazzi L, Rossini M, Adami S, Gatti D (2013) Sclerostin and DKK1 in primary hyperparathyroidism. Calcif Tissue Int 92:324–329
Ardawi MS, Rouzi AA, Al-Sibiani SA, Al-Senani NS, Qari MH, Mousa SA (2012) High serum sclerostin predicts the occurrence of osteoporotic fractures in postmenopausal women: the Center of Excellence for Osteoporosis Research Study. J Bone Miner Res 27:2592–2602
Ardawi MS, Akhbar DH, Alshaikh A, Ahmed MM, Qari MH, Rouzi AA, Ali AY, Abdulrafee AA, Saeda MY (2013) Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone 56:355–362
Szulc P, Boutroy S, Vilayphiou N, Schoppet M, Rauner M, Chapurlat R, Hamann C, Hofbauer LC (2013) Correlates of bone microarchitectural parameters and serum sclerostin levels in men: the STRAMBO study. J Bone Miner Res 28:1760–1770
Mödder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Melton LJ 3rd, Khosla S (2011) Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res 26:373–379
Bhattoa HP, Wamwaki J, Kalina E, Foldesi R, Balogh A, Antal-Szalmas P (2013) Serum sclerostin levels in healthy men over 50 years of age. J Bone Miner Metab 31:579–584
Ikeda Y, Nakajima A, Aiba A, Koda M, Okawa A, Takahashi K, Yamazaki M (2011) Association between serum leptin and bone metabolic markers, and the development of heterotopic ossification of the spinal ligament in female patients with ossification of the posterior longitudinal ligament. Eur Spine J 20:1450–1458
Wada A (1995) Affinity of estrogen binding in the cultured spinal ligament cells: an in vitro study using cells from spinal ligament ossification patients. Nihon Seikeigeka Gakkai Zasshi 69:440–449 (In Japanese)
Ardawi MS, Al-Kadi HA, Rouzi AA, Qari MH (2011) Determinants of serum sclerostin in healthy pre- and postmenopausal women. J Bone Miner Res 26:2812–2822
Sugimori K, Kawaguchi Y, Ohmori K, Kanamori M, Ishihara H, Kimura T (2003) Significance of bone formation markers in patients with ossification of the posterior longitudinal ligament of the spine. Spine 28:378–379
Ishihara C, Kushida K, Takahashi M, Ohishi T, Murata H, Nagano A, Goto S (2000) The efficacy of biochemical markers in patients with ossification of posterior longitudinal ligament of the spine. Spinal Cord 38:211–213
García-Martín A, Rozas-Moreno P, Reyes-García R, Morales-Santana S, García-Fontana B, García-Salcedo JA, Muñoz-Torres M (2012) Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 97:234–241
Morvan F, Boulukos K, Clément-Lacroix P, Roman Roman S, Suc-Royer I, Vayssière B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G (2006) Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 2006:934–945
van Lierop AH, Moester MJ, Hamdy NA, Papapoulos SE (2014) Serum Dickkopf 1 levels in sclerostin deficiency. J Clin Endocrinol Metab 99:E252–E256
Senolt L, Hulejova H, Krystufkova O, Forejtova S, Andres Cerezo L, Gatterova J, Pavelka K, Vencovsky J (2012) Low circulating Dickkopf-1 and its link with severity of spinal involvement in diffuse idiopathic skeletal hyperostosis. Ann Rheum Dis 71:71–74
Aeberli D, Schett G, Eser P, Seitz M, Villiger PM (2011) Serum Dkk-1 levels of DISH patients are not different from healthy controls. Joint Bone Spine 78:422–423
Drake MT, Srinivasan B, Mödder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S (2010) Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95:5056–5062
Murakami M, Seichi A, Chikuda H, Takeshita K, Nakamura K, Kimura A (2010) Long-term follow-up of the progression of ossification of the posterior longitudinal ligament. J Neurosurg Spine 12:577–579
Acknowledgments
Medical editor Katharine O’Moore-Klopf, ELS (East Setauket, New York, USA) provided professional English-language editing of this article before its final acceptance for publication.
Conflict of interest
This work was supported by a Grant-in-aid from the Investigation Committee on Ossification of the Spinal Ligaments, Japanese Ministry of Public Health, Labor. None of the authors has any financial interest with any of the commercial entities mentioned in this article.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kashii, M., Matuso, Y., Sugiura, T. et al. Circulating sclerostin and dickkopf-1 levels in ossification of the posterior longitudinal ligament of the spine. J Bone Miner Metab 34, 315–324 (2016). https://doi.org/10.1007/s00774-015-0671-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-015-0671-5