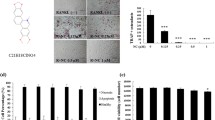
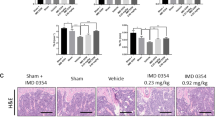
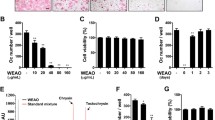
Abstract
1-Alpha, 25-dihydroxy vitamin D3 (1α,25(OH)2D3), an active form of vitamin D3, plays a critical role in calcium and bone metabolism. Although 1α,25(OH)2D3 has been used for osteoporosis therapy, the direct role of 1α,25(OH)2D3 on human osteoclastogenesis has not been well characterized. Here we show that 1α,25(OH)2D3 treatment significantly inhibited human osteoclast formation at the early stage of differentiation in a concentration-dependent manner. 1α,25(OH)2D3 inhibited the expression of nuclear factor of activated T cells c1 (NFATc1, also referred as NFAT2), an essential transcription factor for osteoclast differentiation, and upregulated the expression of interferon-β (IFN-β), a strong inhibitor of osteoclastogenesis in osteoclast progenitors. Inhibitory effects of 1α,25(OH)2D3 on osteoclastogenesis and NFATc1 expression were restored by treatment with an antibody against IFN-β, suggesting that upregulation of IFN-β by 1α,25(OH)2D3 treatment results in inhibition of NFATc1 expression, in turn interfering with osteoclast formation. Thus, our study may provide a molecular basis for the treatment of human bone diseases by 1α,25(OH)2D3 through regulation of the IFN-β and NFATc1 axis.






Similar content being viewed by others
References
Gallagher JC, Riggs BL, Eisman J, Hamstra A, Arnaud SB, DeLuca HF (1979) Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: effect of age and dietary calcium. J Clin Invest 64:729–736
Okazaki T, Igarashi T, Kronenberg HM (1988) 5′-Flanking region of the parathyroid hormone gene mediates negative regulation by 1, 25-(OH)2 vitamin D3. J Biol Chem 263:2203–2208
Demay MB, Kiernan MS, DeLuca HF, Kronenberg HM (1992) Sequences in the human parathyroid hormone gene that bind the 1, 25-dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1, 25-dihydroxyvitamin D3. Proc Natl Acad Sci USA 89:8097–8101
Shiraishi A, Takeda S, Masaki T, Higuchi Y, Uchiyama Y, Kubodera N, Sato K, Ikeda K, Nakamura T, Matsumoto T, Ogata E (2000) Alfacalcidol inhibits bone resorption and stimulates formation in an ovariectomized rat model of osteoporosis: distinct actions from estrogen. J Bone Miner Res 15:770–779
Uchiyama Y, Higuchi Y, Takeda S, Masaki T, Shira-Ishi A, Sato K, Kubodera N, Ikeda K, Ogata E (2002) ED-71, a vitamin D analog, is a more potent inhibitor of bone resorption than alfacalcidol in an estrogen-deficient rat model of osteoporosis. Bone (NY) 30:582–588
Sairanen S, Kärkkäinen M, Tähtelä R, Laitinen K, Mäkelä P, Lamberg-Allardt C, Välimäki MJ (2000) Bone mass and markers of bone and calcium metabolism in postmenopausal women treated with 1, 25-dihydroxyvitamin D (calcitriol) for four years. Calcif Tissue Int 67:122–127
Lukert BP, Raisz LG (1990) Glucocorticoid-induced osteoporosis: pathogenesis and management. Ann Intern Med 112:352–364
Slatopolsky E, Weerts C, Thielan J, Horst R, Harter H, Martin KJ (1984) Marked suppression of secondary hyperparathyroidism by intravenous administration of 1, 25-dihydroxy-cholecalciferol in uremic patients. J Clin Invest 74:2136–2143
Miyamoto T, Suda T (2003) Differentiation and function of osteoclasts. Keio J Med 52:1–7
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature (Lond) 397:315–323
Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J (1999) RANK is essential for osteoclast and lymph node development. Genes Dev 13:2412–2424
Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, Nakao K, Nakamura K, Katsuki M, Yamamoto T, Inoue J (1999) Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 4:353–362
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3:889–901
Johnson RS, Spiegelman BM, Papaioannou V (1992) Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell 71:577–586
Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, Bachler MA, Amano H, Aburatani H, Ishikawa H, Wagner EF (2004) Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem 279:26475–26480
Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, Takayanagi H (2005) Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med 202:1261–1269
Matsuo K, Owens JM, Tonko M, Elliott C, Chambers TJ, Wagner EF (2000) Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nat Genet 24:184–187
Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N, Wagner EF, Taniguchi T (2002) RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature (Lond) 416:744–749
Aloia JF, Vaswani A, Yeh JK, Ellis K, Yasumura S, Cohn SH (1988) Calcitriol in the treatment of postmenopausal osteoporosis. Am J Med 84:401–408
Takasu H, Sugita A, Uchiyama Y, Katagiri N, Okazaki M, Ogata E, Ikeda K (2006) c-Fos protein as a target of anti-osteoclastogenic action of vitamin D, and synthesis of new analogs. J Clin Invest 116:528–535
Iwamoto K, Miyamoto T, Sawatani Y, Hosogane N, Hamaguchi I, Takami M, Nomiyama K, Takagi K, Suda T (2004) Ligand-independent dimer formation of receptor activator of nuclear factor kappa B (RANK) induces incomplete osteoclast formation. Biochem Biophys Res Commun 325:229–234
Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T (2005) DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med 202:345–351
Morita K, Miyamoto T, Fujita N, Kubota Y, Ito K, Takubo K, Miyamoto K, Ninomiya K, Suzuki T, Iwasaki R, Yagi M, Takaishi H, Toyama Y, Suda T (2007) Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J Exp Med 204:1613–1623
Matsumoto T, Miki T, Hagino H, Sugimoto T, Okamoto S, Hirota T, Tanigawara Y, Hayashi Y, Fukunaga M, Shiraki M, Nakamura T (2005) A new active vitamin D, ED-71, increases bone mass in osteoporotic patients under vitamin D supplementation: a randomized, double-blind, placebo-controlled clinical trial. J Clin Endocrinol Metab 90:5031–5036
Takahashi N, Kukita T, MacDonald BR, Bird A, Mundy GR, McManus LM, Miller M, Boyde A, Jones SJ, Roodman GD (1989) Osteoclast-like cells form in long-term human bone marrow but not in peripheral blood cultures. J Clin Invest 83:543–550
Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Giordano T, Gu K, Shah V, Pei L, Zarbo RJ, McCauley L, Shi S, Chen S, Wang CY (2007) NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med 13:62–69
Miyamoto T, Ohneda O, Arai F, Iwamoto K, Okada S, Takagi K, Anderson DM, Suda T (2001) Bifurcation of osteoclasts and dendritic cells from common progenitors. Blood 98:2544–2554
Kitanaka S, Takeyama K, Murayama A, Sato T, Okumura K, Nogami M, Hasegawa Y, Niimi H, Yanagisawa J, Tanaka T, Kato S (1998) Inactivating mutations in the 25-hydroxyvitamin D3 1α-hydroxylase gene in patients with pseudovitamin D-deficiency rickets. N Engl J Med 338:653–661
Koike N, Ichikawa F, Nishii Y, Stumpf E (1998) Sustained osteoblast nuclear receptor binding of converted 1α, 25-dihydroxyvitamin D3 after administration of 3H–1α-hydroxyvitamin D3: a combined receptor autoradiography and radioassay time course study with comparison to 3H–1α, 25-dihydroxyvitamin D3. Calcif Tissue Int 63:391–395
Acknowledgments
We thank Y. Sato for technical support. T. Miyamoto was supported by Precursory Research for Embryonic Science and Technology, Japan Science and Technology Agency, Japan Society for the Promotion of Science Fujita Memorial Fund for Medical Research, and a grant-in-aid from the Global COE Program of the Ministry of Education, Culture, Sports, Science and Technology, Japan, to Keio University.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sakai, S., Takaishi, H., Matsuzaki, K. et al. 1-Alpha, 25-dihydroxy vitamin D3 inhibits osteoclastogenesis through IFN-beta-dependent NFATc1 suppression. J Bone Miner Metab 27, 643–652 (2009). https://doi.org/10.1007/s00774-009-0084-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-009-0084-4