Abstract
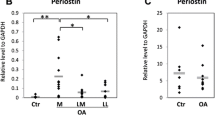
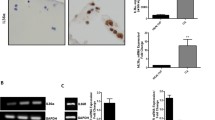
To clarify the significance of the osteophytes that appear during the progression of osteoarthritis (OA), we investigated the expression of inflammatory cytokines and proteases in osteoblasts from osteophytes. We also examined the influence of mechanical stress loading on osteoblasts on the expression of inflammatory cytokines and proteases. Osteoblasts were isolated from osteophytes in 19 patients diagnosed with knee OA and from subchondral bone in 4 patients diagnosed with femoral neck fracture. Messenger RNA expression and protein production of inflammatory cytokines and proteases were analyzed using real-time RT-PCR and ELISA, respectively. To examine the effects of mechanical loading, continuous hydrostatic pressure was applied to the osteoblasts. We determined the mRNA expression and protein production of IL-6, IL-8, and MMP-13, which are involved in the progression of OA, were increased in the osteophytes. Additionally, when OA pathological conditions were simulated by applying a nonphysiological mechanical stress load, the gene expression of IL-6 and IL-8 increased. Our results suggested that nonphysiological mechanical stress may induce the expression of biological factors in the osteophytes and is involved in OA progression. By controlling the expression of these genes in the osteophytes, the progression of cartilage degeneration in OA may be reduced, suggesting a new treatment strategy for OA.








Similar content being viewed by others
References
Bailey AJ, Mansell JP (1997) Do subchondral bone changes exacerbate or precede articular cartilage destruction in osteoarthritis of the elderly? Gerontology 43:296–304
Menkes CJ, Lane NE (2004) Are osteophytes good or bad? Osteoarthritis Cartilage 12(suppl A):S53–S54
Moskowitz RW, Goldberg VM (1987) Studies of osteophyte pathogenesis in experimentally induced osteoarthritis. J Rheumatol 14:311–320
Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR (2005) Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum 52:128–135
Franchimont N, Rydziel S, Delany AM, Canalis E (1997) Interleukin-6 and its soluble receptor cause a marked induction of collagenase 3 expression in rat osteoblast cultures. J Biol Chem 272:12144–12150
Legendre F, Dudhia J, Pujol JP, Bogdanowicz P (2003) JAK/STAT but not ERK1/ERK2 pathway mediates interleukin (IL)-6/soluble IL-6R down-regulation of type II collagen, aggrecan core, and link protein transcription in articular chondrocytes. Association with a down-regulation of SOX9 expression. J Biol Chem 278:2903–2912
Matsukawa A, Yoshimura T, Maeda T, Ohkawara S, Takagi K, Yoshinaga M (1995) Neutrophil accumulation and activation by homologous IL-8 in rabbits. IL-8 induces destruction of cartilage and production of IL-1 and IL-1 receptor antagonist in vivo. J Immunol 154:5418–5425
Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van Wart H, Poole AR (1997) Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest 99:1534–1545
Vankemmelbeke MN, Holen I, Wilson AG, Ilic MZ, Handley CJ, Kelner GS, Clark M, Liu C, Maki RA, Burnett D, Buttle DJ (2001) Expression and activity of ADAMTS-5 in synovium. Eur J Biochem 268:1259–1268
Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ (2005) Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum 52:2386–2395
Diehl P, Schmitt M, Schauwecker J, Eichelberg K, Gollwitzer H, Gradinger R, Goebel M, Preissner KT, Mittelmeier W, Magdolen U (2005) Effect of high hydrostatic pressure on biological properties of extracellular bone matrix proteins. Int J Mol Med 16:285–289
Kleemann RU, Krocker D, Cedraro A, Tuischer J, Duda GN (2005) Altered cartilage mechanics and histology in knee osteoarthritis: relation to clinical assessment (ICRS Grade). Osteoarthritis Cartilage 13:958–963
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16:494–502
Beresford JN, Gallagher JA, Gowen M, Couch M, Poser J, Wood DD, Russell RG (1984) The effects of monocyte-conditioned medium and interleukin 1 on the synthesis of collagenous and non-collagenous proteins by mouse bone and human bone cells in vitro. Biochim Biophys Acta 801:58–65
Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T (1999) Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci 112(pt 20):3519–3527
De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP (2001) Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 44:1928–1942
Khan WS, Adesida AB, Hardingham TE (2007) Hypoxic conditions increase hypoxia-inducible transcription factor 2-alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res Ther 9:R55
Inoue A, Takahashi KA, Arai Y, Tonomura H, Sakao K, Saito M, Fujioka M, Fujiwara H, Tabata Y, Kubo T (2006) The therapeutic effects of basic fibroblast growth factor contained in gelatin hydrogel microspheres on experimental osteoarthritis in the rabbit knee. Arthritis Rheum 54:264–270
Takahashi K, Kubo T, Arai Y, Kitajima I, Takigawa M, Imanishi J, Hirasawa Y (1998) Hydrostatic pressure induces expression of interleukin 6 and tumour necrosis factor alpha mRNAs in a chondrocyte-like cell line. Ann Rheum Dis 57:231–236
Ahn SE, Kim S, Park KH, Moon SH, Lee HJ, Kim GJ, Lee YJ, Park KH, Cha KY, Chung HM (2006) Primary bone-derived cells induce osteogenic differentiation without exogenous factors in human embryonic stem cells. Biochem Biophys Res Commun 340:403–408
Robbins JR, Thomas B, Tan L, Choy B, Arbiser JL, Berenbaum F, Goldring MB (2000) Immortalized human adult articular chondrocytes maintain cartilage-specific phenotype and responses to interleukin-1-beta. Arthritis Rheum 43:2189–2201
Bustin SA (2002) Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol 29:23–39
Sakao K, Takahashi KA, Arai Y, Inoue A, Tonomura H, Saito M, Yamamoto T, Kanamura N, Imanishi J, Mazda O, Kubo T (2008) Induction of chondrogenic phenotype in synovium-derived progenitor cells by intermittent hydrostatic pressure. Osteoarthritis Cartilage 16:805–814
Legendre F, Bogdanowicz P, Boumediene K, Pujol JP (2005) Role of interleukin 6 (IL-6)/IL-6R-induced signal tranducers and activators of transcription and mitogen-activated protein kinase/extracellular. J Rheumatol 32:1307–1316
Gilbertson EM (1975) Development of periarticular osteophytes in experimentally induced osteoarthritis in the dog. A study using microradiographic, microangiographic, and fluorescent bone-labelling techniques. Ann Rheum Dis 34:12–25
Buckland-Wright JC, Lynch JA, Dave B (2000) Early radiographic features in patients with anterior cruciate ligament rupture. Ann Rheum Dis 59:641–646
Williams JA, Thonar EJ (1989) Early osteophyte formation after chemically induced articular cartilage injury. Am J Sports Med 17:7–15
Ozdemir F, Tukenmez O, Kokino S, Turan FN (2006) How do marginal osteophytes, joint space narrowing and range of motion affect each other in patients with knee osteoarthritis. Rheumatol Int 26:516–522
Nagaosa Y, Lanyon P, Doherty M (2002) Characterisation of size and direction of osteophyte in knee osteoarthritis: a radiographic study. Ann Rheum Dis 61:319–324
Gelse K, Soder S, Eger W, Diemtar T, Aigner T (2003) Osteophyte development—molecular characterization of differentiation stages. Osteoarthritis Cartilage 11:141–148
Sweet MB, Thonar EJ, Immelman AR, Solomon L (1977) Biochemical changes in progressive osteoarthrosis. Ann Rheum Dis 36:387–398
Kaneko S, Satoh T, Chiba J, Ju C, Inoue K, Kagawa J (2000) Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther 6:71–79
Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE (1996) Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest 97:761–768
Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, Yu Y, Fosang AJ, Schorpp-Kistner M, Angel P, Werb Z (2004) Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development (Camb) 131:5883–5895
Tchetina EV, Kobayashi M, Yasuda T, Meijers T, Pidoux I, Poole AR (2007) Chondrocyte hypertrophy can be induced by a cryptic sequence of type II collagen and is accompanied by the induction of MMP-13 and collagenase activity: Implications for development and arthritis. Matrix Biol 26:247–258
Cecil DL, Rose DM, Terkeltaub R, Liu-Bryan R (2005) Role of interleukin-8 in PiT-1 expression and CXCR1-mediated inorganic phosphate uptake in chondrocytes. Arthritis Rheum 52:144–154
Poli V, Balena R, Fattori E, Markatos A, Yamamoto M, Tanaka H, Ciliberto G, Rodan GA, Costantini F (1994) Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J 13:1189–1196
Ferrari SL, Garnero P, Emond S, Montgomery H, Humphries SE, Greenspan SL (2001) A functional polymorphic variant in the interleukin-6 gene promoter associated with low bone resorption in postmenopausal women. Arthritis Rheum 44:196–201
Torres L, Dunlop DD, Peterfy C, Guermazi A, Prasad P, Hayes KW, Song J, Cahue S, Chang A, Marshall M, Sharma L (2006) The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage 14:1033–1040
Boegard T, Rudling O, Petersson IF, Jonsson K (1998) Correlation between radiographically diagnosed osteophytes and magnetic resonance detected cartilage defects in the tibiofemoral joint. Ann Rheum Dis 57:401–407
Felson DT, Gale DR, Elon Gale M, Niu J, Hunter DJ, Goggins J, Lavalley MP (2005) Osteophytes and progression of knee osteoarthritis. Rheumatology (Oxf) 44:100–104
Pottenger LA, Phillips FM, Draganich LF (1990) The effect of marginal osteophytes on reduction of varus–valgus instability in osteoarthritic knees. Arthritis Rheum 33:853–858
Hodge WA, Fijan RS, Carlson KL, Burgess RG, Harris WH, Mann RW (1986) Contact pressures in the human hip joint measured in vivo. Proc Natl Acad Sci USA 83:2879–2883
de la Torre P, Diaz-Sanjuan T, Garcia-Ruiz I, Esteban E, Canga F, Munoz-Yague T, Solis-Herruzo JA (2005) Interleukin-6 increases rat metalloproteinase-13 gene expression through Janus kinase-2-mediated inhibition of serine/threonine phosphatase-2A. Cell Signal 17:427–435
Honorati MC, Bovara M, Cattini L, Piacentini A, Facchini A (2002) Contribution of interleukin 17 to human cartilage degradation and synovial inflammation in osteoarthritis. Osteoarthritis Cartilage 10:799–807
Dai SM, Shan ZZ, Nishioka K, Yudoh K (2005) Implication of interleukin 18 in production of matrix metalloproteinases in articular chondrocytes in arthritis: direct effect on chondrocytes may not be pivotal. Ann Rheum Dis 64:735–742
Wu W, Billinghurst RC, Pidoux I, Antoniou J, Zukor D, Tanzer M, Poole AR (2002) Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum 46:2087–2094
Hulejova H, Baresova V, Klezl Z, Polanska M, Adam M, Senolt L (2007) Increased level of cytokines and matrix metalloproteinases in osteoarthritic subchondral bone. Cytokine 38:151–156
Acknowledgments
We thank Dr. Kawano and Dr. Aoshiba for supplying the clinical specimens. No research or institutional support was received for this work.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sakao, K., Takahashi, K.A., Arai, Y. et al. Osteoblasts derived from osteophytes produce interleukin-6, interleukin-8, and matrix metalloproteinase-13 in osteoarthritis. J Bone Miner Metab 27, 412–423 (2009). https://doi.org/10.1007/s00774-009-0058-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-009-0058-6