Abstract
Epithelial–mesenchymal transition (EMT) of tubular epithelial cells is a key event in renal interstitial fibrosis and the progression of chronic kidney disease (CKD). Apelin is a regulatory peptide involved in the regulation of normal renal hemodynamics and tubular functions, but its role in renal fibrosis remains unknown. In this study, we examined the inhibitory effects of apelin on transforming growth factor-β1 (TGF-β1)-induced EMT in HK-2 cells, and evaluated its therapeutic efficacy in mice with complete unilateral ureteral obstruction (UUO). In vitro, apelin inhibited TGF-β1-mediated upregulation of α-smooth muscle actin (α-SMA) and downregulation of E-cadherin. Increased levels of phosphorylated Smad-2/3 and decreased levels of Smad7 in TGF-β1-stimulated cells were reversed by apelin co-treatment. In the UUO model, administration of apelin significantly attenuated renal interstitial fibrosis, as evidenced by the maintenance of E-cadherin and laminin expression, and markedly suppressed expression of α-SMA, TGF-β1 and its type I receptor, as well as interstitial matrix components. Interestingly, in UUO mice, there was a reduction in the plasma level of apelin, which was compensated by upregulation of APJ expression in the injured kidney. Exogenous supplementation of apelin normalized the level of plasmatic apelin and renal APJ. In conclusion, our study provides the first evidence that apelin is able to ameliorate renal interstitial fibrosis by suppression of tubular EMT through a Smad-dependent mechanism. The apelinergic system itself may promote some compensatory response in the renal fibrotic process. These results suggest that apelin has potential renoprotective effects and may be an effective agent for retarding CKD progression.







Similar content being viewed by others
References
Berry MF, Pirolli TJ, Jayasankar V et al (2004) Apelin has in vivo inotropic effects on normal and failing hearts. Circulation 110:II 187–II 193
Cayabyab M, Hinuma S, Farzan M et al (2000) Apelin, the natural ligand of the orphan seven-transmembrane receptor APJ, inhibits human immunodeficiency virus type 1 entry. J Virol 74:11972–11976
Codognotto M, Piccoli A, Zaninotto M et al (2007) Evidence for decreased circulating apelin beyond heart involvement in uremic cardiomyopathy. Am J Nephrol 27:1–6
Compagnone A, Bandino A, Meli F et al (2012) Polyamines modulate epithelial-to-mesenchymal transition. Amino Acids 42:783–789
Coresh J, Selvin E, Stevens LA et al (2007) Prevalence of chronic kidney disease in the United States. JAMA 298:2038–2047
Cui RR, Mao DA, Yi L et al (2010) Apelin suppresses apoptosis of human vascular smooth muscle cells via APJ/PI3-K/Akt signaling pathways. Amino Acids 39:1193–1200
Das S, Becker BN, Hoffmann FM, Mertz JE (2009) Complete reversal of epithelial to mesenchymal transition requires inhibition of both ZEB expression and the Rho pathway. BMC Cell Biol 10:94
Day RT, Cavaglieri RC, Feliers D (2013) Apelin retards the progression of diabetic nephropathy. Am J Physiol Renal Physiol 304:F788–F800
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425:577–584
El-Shehaby AM, El-Khatib MM, Battah AA, Roshdy AR (2010) Apelin: a potential link between inflammation and cardiovascular disease in end stage renal disease patients. Scand J Clin Lab Invest 70:421–427
Falcao-Pires I, Goncalves N, Henriques-Coelho T et al (2009) Apelin decreases myocardial injury and improves right ventricular function in monocrotaline-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 296:H2007–H2014
Foldes G, Horkay F, Szokodi I et al (2003) Circulating and cardiac levels of apelin, the novel ligand of the orphan receptor APJ, in patients with heart failure. Biochem Biophys Res Commun 308:480–485
He W, Dai C, Li Y et al (2009) Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20:765–776
Hus-Citharel A, Bouby N, Frugiere A et al (2008) Effect of apelin on glomerular hemodynamic function in the rat kidney. Kidney Int 74:486–494
Itoh S, Ten DP (2007) Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol 19:176–184
Jones LK, O’Sullivan KM, Semple T et al (2009) IL-1RI deficiency ameliorates early experimental renal interstitial fibrosis. Nephrol Dial Transplant 24:3024–3032
Kaneyama T, Kobayashi S, Aoyagi D, Ehara T (2010) Tranilast modulates fibrosis, epithelial–mesenchymal transition and peritubular capillary injury in unilateral ureteral obstruction rats. Pathology 42:564–573
Kawamata Y, Habata Y, Fukusumi S et al (2001) Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta 1538:162–171
Lan HY (2003) Tubular epithelial–myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr Opin Nephrol Hypertens 12:25–29
Li Y, Yang J, Dai C, Wu C, Liu Y (2003) Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest 112:503–516
Liu Y (2004) Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15:1–12
Liu Y (2006) Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 69:213–217
Lu Y, Zhu X, Liang GX et al (2012) Apelin-APJ induces ICAM-1, VCAM-1 and MCP-1 expression via NF-kappaB/JNK signal pathway in human umbilical vein endothelial cells. Amino Acids 43:2125–2136
Medhurst AD, Jennings CA, Robbins MJ et al (2003) Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J Neurochem 84:1162–1172
Melgar-Lesmes P, Casals G, Pauta M et al (2010) Apelin mediates the induction of profibrogenic genes in human hepatic stellate cells. Endocrinology 151:5306–5314
Najafipour H, Soltani HA, Nekooian AA, Esmaeili-Mahani S (2012) Apelin receptor expression in ischemic and non- ischemic kidneys and cardiovascular responses to apelin in chronic two-kidney-one-clip hypertension in rats. Regul Pept 178:43–50
Nishida M, Okumura Y, Oka T et al (2012) The role of apelin on the alleviative effect of Angiotensin receptor blocker in unilateral ureteral obstruction-induced renal fibrosis. Nephron Extra 2:39–47
Pchejetski D, Foussal C, Alfarano C et al (2012) Apelin prevents cardiac fibroblast activation and collagen production through inhibition of sphingosine kinase 1. Eur Heart J 33:2360–2369
Principe A, Melgar-Lesmes P, Fernandez-Varo G et al (2008) The hepatic apelin system: a new therapeutic target for liver disease. Hepatology 48:1193–1201
Siddiquee K, Hampton J, Khan S et al (2011) Apelin protects against angiotensin II-induced cardiovascular fibrosis and decreases plasminogen activator inhibitor type-1 production. J Hypertens 29:724–731
Sorhede WM, Magnusson C, Ahren B (2005) The apj receptor is expressed in pancreatic islets and its ligand, apelin, inhibits insulin secretion in mice. Regul Pept 131:12–17
Strutz FM (2009) EMT and proteinuria as progression factors. Kidney Int 75:475–481
Sun S, Ning X, Zhang Y et al (2009) Hypoxia-inducible factor-1alpha induces Twist expression in tubular epithelial cells subjected to hypoxia, leading to epithelial-to-mesenchymal transition. Kidney Int 75:1278–1287
Ten DP, Hill CS (2004) New insights into TGF-beta-Smad signalling. Trends Biochem Sci 29:265–273
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214:199–210
Yang J, Liu Y (2002) Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J Am Soc Nephrol 13:96–107
Zeisberg M, Hanai J, Sugimoto H (2003) BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9:964–968
Acknowledgments
This study received financial support from the National Natural Science Foundation of the People’s Republic of China (81300607), the Beijing Natural Science Foundation (7132091), Beijing Municipal Science and Technology Commission Funds (D131100004713001; Z121107001012138), and Capital Health Research and Development Project (2011-2002-02).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
726_2014_1826_MOESM1_ESM.doc
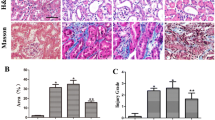
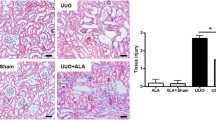
Supplementary material 1 (DOC 285 kb) Figure S1. Apelin inhibits TGF-β-induced EMT and attenuates renal interstitial fibrosis through the APJ receptor. (A-C) Human proximal tubular epithelial cells (HK-2) treated with TGF-β1 (2 ng/ml) or/and apelin-13 (1 µmol/L) were exposed to F13A (2 µmol/L), a competitive antagonist for APJ, for 48 h. Western blotting showed that the restored expression of E-cadherin and decreased expression of α-SMA in HK-2 cells co-treated with TGF-β1 and apelin-13 were significantly reversed by F13A. Cell lysates were immunoblotted with antibodies against α-SMA, E-cadherin, or β-actin. Results are presented as percentages of control values after normalization to β-actin and are the means ± SD of three independent experiments. *P < 0.05, compared with control groups. # P < 0.05, compared with TGF-β1-stimulated groups. & P < 0.05, compared with TGF-β1 and apelin-13 co-treatment group, n = 5 in each group. (D and E) Representative micrographs showing that F13A (0.2 µmol/kg/d) suppressed the antifibrotic effects of apelin-13 (0.1 µmol/kg/d) in UUO mice. Kidney sections from various groups at 14 days after UUO were subjected to MTS. Quantitative determination of renal fibrotic lesions in various groups is shown. Renal fibrotic lesions (defined as the percentage of the MTS-positive fibrotic area) were quantified by computer-aided morphometric analyses. Results are the means ± SD of five animals per group (n = 5). *P < 0.05, compared with the UUO− apelin− F13A− group. # P < 0.05, compared with the UUO+ apelin− F13A− group. & P < 0.05, compared with the UUO+ apelin+ F13A− group. Scale bar, 40 μm
Rights and permissions
About this article
Cite this article
Wang, LY., Diao, ZL., Zhang, DL. et al. The regulatory peptide apelin: a novel inhibitor of renal interstitial fibrosis. Amino Acids 46, 2693–2704 (2014). https://doi.org/10.1007/s00726-014-1826-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1826-8