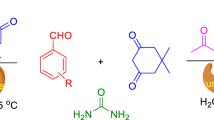
Abstract
Bovine serum albumin (BSA) promoted simple and efficient one-pot procedure was developed for the direct synthesis of 3,4-dihydropyrimidin-2(1H)-ones including potent mitotic kinesin Eg5 inhibitor monastrol under mild reaction conditions. The catalyst recyclability and gram scale synthesis have also been demonstrated to enhance the practical utility of process.


Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- CAL-B:
-
Candida antarctica lipase-B
- DHPMs:
-
3,4-dihydropyrimidin-2(1H)-ones
- HIV:
-
Human immunodeficiency virus
- HPLC:
-
High-performance liquid chromatography
- PPL:
-
Porcine pancreas lipase
References
Banik BK, Reddy AT, Datta A, Mukhopadhyay C (2007) Microwave-induced bismuth nitrate-catalyzed synthesis of dihydropyrimidones via Biginelli condensation under solventless conditions. Tetrahedron Lett 48:7392–7394
Bhosale RS, Bhosale SV, Bhosale SV, Wang T, Zubaidha PK (2004) An efficient, high yield protocol for the one-pot synthesis of dihydropyrimidin-2(1H)-ones catalyzed by iodine. Tetrahedron Lett 45:9111–9113
Biggs-Houck JE, Younai A, Shaw JT (2010) Recent advances in multicomponent reactions for diversity- oriented synthesis. Curr Opin Chem Biol 14:371–382
Biginelli P (1893) Aldehyde-urea derivatives of aceto- and oxaloacetic acids. Gazz Chim Ital 23:360-413
Borse BN, Borude VS, Shukla SR (2012) Synthesis of novel dihydropyrimidin-2(1H)-ones derivatives using lipase and their antimicrobial activity. Curr Chem Lett 1:59–68
Bose AK, Pednekar S, Ganguly SN, Chakraborty G, Manhas MS (2004) A simplified green chemistry approach to the Biginelli reaction using ‘Grindstone Chemistry’. Tetrahedron Lett 45:8351–8353
Boucher G, Sylvain R, Fargeas V, Dintinger T, Mathe-Allainmat M, Lebreton J, Tellier C (2005) Serum albumin-catalyzed trigger system by using a tandem kemp elimination/b-elimination reaction. ChemBioChem 6:807–810
Chari MA, Mano A, Selvan ST, Mukkanti K, Vinu A (2009) Synthesis of 3,4-dihydropyrimidin-2-ones (DHPMs) using mesoporous aluminosilicate (AlKIT-5) catalyst with cage type pore structure. Tetrahedron 65:10608–10611
Chitra S, Pandiarajan K (2009) Calcium fluoride: an efficient and reusable catalyst for the synthesis of 3,4- dihydropyrimidin-2(1H)-ones and their corresponding 2(1H)thione: an improved high yielding protocol for the Biginelli reaction. Tetrahedron Lett 50:2222–2224
Clouthier CM, Pelletier JN (2012) Expanding the organic toolbox: a guide to integrating biocatalysis in synthesis. Chem Soc Rev 41:1585–1605
Debache A, Amimour M, Belfaitah A, Rhouati S, Carboni B (2008) A one-pot Biginelli synthesis of 3,4- dihydropyrimidin-2-(1H)-ones/thiones catalyzed by triphenylphosphine as Lewis base. Tetrahedron Lett 49:6119–6121
Debache A, Boumoud B, Amimour M, Belfaitah A, Rhouati S, Carboni B (2006) Phenylboronic acid as a mild and efficient catalyst for Biginelli reaction. Tetrahedron Lett 47:5697–5699
De Souza ROMA, da Penha ET, Milagre HMS, Garden SJ, Esteves PM, Eberlin MN, Antunes OAC (2009) The three-component Biginelli reaction: a combined experimental and theoretical mechanistic investigation. Chem Eur J 15:9799–9804
Dömling A (2006) Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev 106:17–89
Gore S, Baskaran S, Koenig B (2011) Efficient synthesis of 3,4-dihydropyrimidin-2-ones in low melting tartaric acid–urea mixtures. Green Chem 13:1009–1013
Hojati SF, Gholizadeh M, Haghdoust M, Shafiezadeh F (2010) 1,3-Dichloro-5,5-dimethylhydantoin as a novel and efficient homogeneous catalyst in Biginelli reaction. Bull Kor Chem Soc 31:3238–3240
Hollfelder F, Kirby AJ, Tawfik DS (1996) Off-the-shelf proteins that rival tailor-made antibodies as catalysts. Nature 383:60–63
Hudlicky T, Reed JW (2009) Applications of biotransformations and biocatalysis to complexity generation in organic synthesis. Chem Soc Rev 38:3117–3132
Jain KS, Bariwal JB, Kathiravan MK, Phoujdar MS, Sahne RS, Chauhan BS, Shah AK, Yadav MR (2008) Recent advances in selective a1-adrenoreceptor antagonists as antihypertensive agents. Bioorg Med Chem 16:4759–4800
Kappe CO (1997) A re-examination of the mechanism of the Biginelli dihydropyrimidine synthesis. Support for an N-acyliminium ion intermediate. J Org Chem 62:7201–7204
Kappe CO (1998) 4-Aryldihydropyrimidines via the Biginelli condensation: aza analogs of nifedipine-type calcium channel modulators. Molecules 3:1–9
Kappe CO (2000) Biologically active dihydropyrimidones of the Biginelli-type—a literature survey. Eur J Med Chem 35:1043–1052
Kasana RC, Sharma UK, Sharma N, Sinha AK (2007) Isolation and identification of a novel strain of Pseudomonas chlororaphis capable of transforming isoeugenol to vanillin. Curr Microbiol 54:457–461
Kidwai M, Saxena S, Khan MKR, Thukral SS (2005) Synthesis of 4-aryl-7,7-dimethyl-1,2,3,4,5,6,7,8- octahydroquinazoline-2-one/thione-5-one derivatives and evaluation as antibacterials. Eur J Med Chem 40:816–819
Klein G, Reymond JM (1998) An enantioselective fluorimetric assay for alcohol dehydrogenases using albumin-catalyzed-elimination of umbelliferone. Bioorg Med Chem Lett 8:1113–1116
Kumar A, Maurya RA (2007) An efficient bakers’ yeast catalyzed synthesis of 3,4-dihydropyrimidin-2-(1H)-ones. Tetrahedron Lett 48:4569–4571
Kumar P, Sankar BR, Nasir G, Baig RB, Chandrashekaran S (2009) Novel Biginelli dihydropyrimidines with potential anticancer activity: a parallel synthesis and CoMSIA study. Eur J Med Chem 44:4192–4198
Lannou MI, Hélion F, Namy JL (2008) Applications of lanthanide trichloride hydrates, prepared from mischmetall, in the Biginelli reaction. Synlett 105-107
Li JT, Han JF, Yang JH, Li TS (2003) An efficient synthesis of 3,4-dihydropyrimidin-2-ones catalyzed by NH2SO3H under ultrasound irradiation. Ultrasound Sonochem 10:119–122
Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ (1999) Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286:971–974
Norberto DR, Vieira JM, de Souza AR, Bispo JA, Bonafe CFS (2012) Pressure & urea-induced denaturation of bovine serum albumin: considerations about protein heterogeneity. Open J Biophys 2:4–14
Patel RN (2008) Synthesis of chiral pharmaceutical intermediates by biocatalysis. Coord Chem Rev 252:659–701
Pollard DJ, Woodley JM (2007) Biocatalysis for pharmaceutical intermediates: the future is now. Trends Biotechnol 25:66–73
Polshettiwar V, Varma RS (2007) Biginelli reaction in aqueous medium: a greener and sustainable approach to substituted 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett 48:7343–7346
Prasad AK, Arya P, Bhatia S, Sharma RK, Singh R, Singh BK, Van der Eycken E, Singh R, Olsen CE, Parmar VS (2009) Synthesis and lipase-catalysed enantioselective acylation studies on Ethyl-4-aryl-3,4- dihydropyrimidin-2(1H)-ones. Ind J Chem 48B:1738–1748
Rafiee E, Jafari H (2006) A practical and green approach towards synthesis of dihydropyrimidinones: using heteropoly acids as efficient catalysts. Bioorg Med Chem Lett 16:2463–2466
Ramalingan C, Park S-J, Lee I-S, Kwak Y-W (2010) A piperidinium triflate catalyzed Biginelli reaction. Tetrahedron 66:2987–2994
Reetz MT, Mondire R, Carballeira JD (2007) Enzyme promiscuity: first protein-catalyzed Morita–Baylis–Hillman reaction. Tetrahedron Lett 48:1679–1681
Riva S, Mendozza M, Carrea G, Chattopadhay P, Tramontano A (1998) Comparison of antibody and albumin catalyzed hydrolysis of steroidal p-Nitrophenylcarbonates. Appl Biochem Biotechnol 75:33–44
Saha S, Moorthy JN (2011) Enantioselective organocatalytic Biginelli reaction: dependence of the catalyst on Sterics, hydrogen bonding, and reinforced chirality. J Org Chem 76:396–402
Santacoloma PA, Sin G, Gernaey KV, Woodley JM (2011) Multienzyme-catalyzed processes: next- generation biocatalysis. Org Process Res Develop 15:203–212
Schmid A, Dordick JS, Hauer B, Kiener A, Wubbolts M, Witholt B (2001) Industrial biocatalysis today and tomorrow. Nature 409:258–268
Schnell B, Krenn W, Faber K, Kappe CO (2000) Synthesis and reactions of Biginelli-compounds. Part 23. chemoenzymatic syntheses of enantiomerically pure 4-aryl-3,4-dihydropyrimidin-2(1H)-ones. J Chem Soc Perkin Trans 1:4382–4389
Sharma N, Sharma UK, Salwan R, Kasana RC, Sinha AK (2011a) A synergic blend of newly isolated Pseudomonas mandelii KJLPB5 and [hmim]Br for chemoselective 2º aryl alcohol oxidation in H2O2: synthesis of aryl ketone or aldehydes via sequential dehydration-oxidative C = C cleavage. Catal Lett 141:616–622
Sharma N, Sharma UK, Kumar R, Kumar R, Katoch N, Sinha AK (2011b) First bovine serum albumin- promoted synthesis of enones, cinnamic acids and coumarins in ionic liquid: an insight into the role of protein impurities in porcine pancreas lipase for olefinic bond formation. Adv Synth Catal 353:871–878
Sharma N, Sharma UK, Kumar R, Richa AK, Sinha AK (2012a) Green and recyclable glycine nitrate (GlyNO3) ionic liquid triggered multicomponent Biginelli reaction for the efficient synthesis of dihydropyrimidinones. RSC Adv 2:10648–10651
Sharma UK, Sharma N, Kumar R, Kumar R, Sinha AK (2009) Biocatalytic promiscuity of lipase in chemoselective oxidation of aryl alcohols/acetates: a unique synergism of CAL-B and [hmim]Br for the metal-free H2O2 activation. Org Lett 11:4846–4848
Sharma UK, Sharma N, Salwan R, Kumar R, Kasana RC, Sinha AK (2012b) Efficient synthesis of hydroxystyrenes via biocatalytic decarboxylation/deacetylation of substituted cinnamic acids by newly isolated Pantoea agglomerans strains. J Sci Food Agric 92:610–616
Shen ZL, Xu XP, Ji SJ (2010) Brønsted base-catalyzed one-pot three-component Biginelli-type reaction: an efficient synthesis of 4,5,6-triaryl-3,4-dihydropyrimidin-2(1H)-one and mechanistic study. J Org Chem 75:1162–1167
Strohmeier GA, Sovic T, Steinkellner G, Hartner FS, Andryushkova A, Purkarthofer T, Glieder A, Gruber K, Griengl H (2009) Investigation of lipase-catalyzed Michael-type carbon–carbon bond formations. Tetrahedron 65:5663–5668
Suzuki I, Iwata Y, Takeda K (2008) Biginelli reactions catalyzed by hydrazine type organocatalyst. Tetrahedron Lett 49:3238–3241
Tamaddon F, Razmi Z, Jafari AA (2010) Synthesis of 3,4-dihydropyrimidin-2(1H)-ones and 1,4-dihydropyridines using ammonium carbonate in water. Tetrahedron Lett 51:1187–1189
Taylor RP, Chau V, Bryner C, Berga S (1975) Bovine serum albumin as a catalyst. II. Characterization of the kinetics. J Am Chem Soc 97:1934–1943
Van Rantwijk F, Sheldon RA (2007) Biocatalysis in ionic liquids. Chem Rev 107:2757–2785
Walker JM (ed) (2005) Protein identification and analysis tools on the ExPASy server. The proteomics protocols handbook. Humana press, Totowa, pp 571–607
Wang J-L, Liu B-K, Yin C, Wu Q, Lin X-F (2011) Candida antarctica lipase B-catalyzed the unprecedented three-component Hantzsch-type reaction of aldehyde with acetamide and 1,3-dicarbonyl compounds in non-aqueous solvent. Tetrahedron 67:2689–2692
Wipf P, Stephenson CRJ, Okumura K (2003) Transition-metal-mediated cascade reactions: C, C-dicyclopropylmethylamines by way of double C, C-σ-bond insertion into bicyclobutanes. J Am Chem Soc 125:14694–14695
Yarim M, Saraç S, Kiliç FS, Erol K (2003) Synthesis and in vitro calcium antagonist activity of 4-aryl-7,7-dimethyl/1,7,7-trimethyl-1,2,3,4,5,6,7,8-octahydroquinazoline-2,5-dione derivatives. Farmaco 58:17–24
Yu Y, Liu C, Luo G (2007) One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using chloroacetic acid as catalyst. Bioorg Med Chem Lett 17:3508–3510
Zhu J, Bienaymé H (2005) Multicomponent reactions. Wiley-VCH, Weinheim
Znabet A, Polak MM, Janssen E, de Kanter FJJ, Turner NJ, Orru RVA, Ruijter E (2010a) A highly efficient synthesis of telaprevir by strategic use of biocatalysis and multicomponent reactions. Chem Commun 46:7918–7920
Znabet A, Zonneveld J, Janssen E, de Kanter FJJ, Helliwell M, Turner NJ, Ruijter E, Orru RVA (2010b) Asymmetric synthesis of synthetic alkaloids by a tandem biocatalysis/Ugi/Pictet–Spengler-type cyclization sequence. Chem Commun 46:7706–7708
Acknowledgments
UKS, NS, RK are indebted to CSIR, New Delhi, for the award of research fellowships. The authors gratefully acknowledge the Director, IHBT Palampur, for his kind cooperation and encouragement as well as project MLP0025 for financial assistance. IHBT Communication No. 2371.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, U.K., Sharma, N., Kumar, R. et al. Biocatalysts for multicomponent Biginelli reaction: bovine serum albumin triggered waste-free synthesis of 3,4-dihydropyrimidin-2-(1H)-ones. Amino Acids 44, 1031–1037 (2013). https://doi.org/10.1007/s00726-012-1437-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1437-1