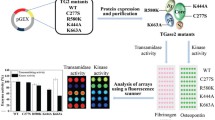
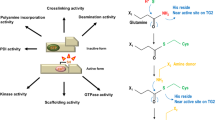
Abstract
Transglutaminase 2 (TG2) is a multifunctional member of an enzyme family: it modifies glutamine residues by cross-linking proteins and incorporating primary amines into them, has protein disulphide isomerase and protein kinase activities, mediates trans-membrane signal transduction and interactions between cell surface proteins and the extracellular matrix. These unusual multiple roles encoded into one polypeptide chain suggest that genomic variations in the TGM2 gene should be limited. Indeed, the available information in databases shows that unlike in the case of most other transglutaminases there are no common single nucleotide polymorphisms in exons of human TGM2. We collected data on and produced some of the rare genetic variants of TGM2 by site directed mutagenesis and found that some were less stable than the most abundant (wild type) enzyme variant and the majority had deficient transamidating activity. Further studies are required to clarify the pathologic significance of these rare TGM2 alleles in the human population.





Similar content being viewed by others
References
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR (2010) A method and server for predicting damaging missense mutations. Nat Methods 7:248–249
Anwar R, Gallivan L, Edmonds SD, Markham AF (1999) Genotype/phenotype correlations for coagulation factor XIII: specific normal polymorphisms are associated with high or low factor XIII specific activity. Blood 93:897–905
Bernassola F, Federici M, Corazzari M, Terrinoni A, Hribal ML, De Laurenzi V, Ranalli M, Massa O, Sesti G, McLean WH, Citro G, Barbetti F, Melino G (2002) Role of transglutaminase 2 in glucose tolerance: knockout mice studies and a putative mutation in a MODY patient. FASEB J 16:1371–1378
Bradford M, Law MH, Stewart AD, Shaw DJ, Megson IL, Wei J (2009) The TGM2 gene is associated with schizophrenia in a British population. Am J Med Genet B Neuropsychiatr Genet 150B:335–340
Cassidy AJ, van Steensel MA, Steijlen PM, van Geel M, van der Velden J, Morley SM, Terrinoni A, Melino G, Candi E, McLean WH (2005) A homozygous missense mutation in TGM5 abolishes epidermal transglutaminase 5 activity and causes acral peeling skin syndrome. Am J Hum Genet 77:909–917
Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, Handsaker RE, Lunter G, Marth GT, Sherry ST, McVean G, Durbin R, 1000 Genomes Project Analysis Group (2011) The variant call format and VCFtools. Bioinformatics 27:2156–2158
Duckert F, Jung E, Shmerling DH (1960) A hitherto undescribed congenital haemorrhagic diathesis probably due to fibrin stabilizing factor deficiency. Thromb Diath Haemorrh 5:179–186
Drmanac R, Sparks AB, Callow MJ, Halpern AL, Burns NL, Kermani BG, Carnevali P, Nazarenko I, Nilsen GB, Yeung G, Dahl F, Fernandez A, Staker B, Pant KP, Baccash J, Borcherding AP, Brownley A, Cedeno R, Chen L, Chernikoff D, Cheung A, Chirita R, Curson B, Ebert JC, Hacker CR, Hartlage R, Hauser B, Huang S, Jiang Y, Karpinchyk V, Koenig M, Kong C, Landers T, Le C, Liu J, McBride CE, Morenzoni M, Morey RE, Mutch K, Perazich H, Perry K, Peters BA, Peterson J, Pethiyagoda CL, Pothuraju K, Richter C, Rosenbaum AM, Roy S, Shafto J, Sharanhovich U, Shannon KW, Sheppy CG, Sun M, Thakuria JV, Tran A, Vu D, Zaranek AW, Wu X, Drmanac S, Oliphant AR, Banyai WC, Martin B, Ballinger DG, Church GM, Reid CA (2010) Human genome sequencing using unchained base reads on self-assembling DNA nanoarrays. Science 327:78–81
Fesus L, Piacentini M (2002) Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem Sci 27:534–539
Folk JE, Cole PW (1966) Mechanism of action of guinea pig liver transglutaminase. I. Purification and properties of the enzyme: identification of a functional cysteine essential for activity. J Biol Chem 241:5518–5525
Folk JE, Chung SI (1973) Molecular and catalytic properties of transglutaminases. Adv Enzymol Relat Areas Mol Biol 38:109–191
Galperin MY, Cochrane GR (2011) The 2011 nucleic acids research database issue and the online molecular biology database collection. Nucl Acids Res 39(Database issue):D1–D6
Gentile V, Saydak M, Chiocca EA, Akande O, Birckbichler PJ, Lee KN, Stein JP, Davies PJ (1991) Isolation and characterization of cDNA clones to mouse macrophage and human endothelial cell tissue transglutaminases. J Biol Chem 266:478–483
Huber M, Rettler I, Bernasconi K, Frenk E, Lavrijsen SP, Ponec M, Bon A, Lautenschlager S, Schorderet DF, Hohl D (1995) Mutations of keratinocyte transglutaminase in lamellar ichthyosis. Science 267:525–528
Karimi M, Bereczky Z, Cohan N, Muszbek L (2009) Factor XIII deficiency. Semin Thromb Hemost 35:426–438
Kharfi M, El Fekih N, Ammar D, Jaafoura H, Schwonbeck S, van Steensel MA, Fazaa B, Kamoun MR, Fischer J (2009) A missense mutation in TGM5 causes acral peeling skin syndrome in a Tunisian family. J Invest Dermatol 129:2512–2515
Király R, Vecsei Z, Deményi T, Korponay-Szabó IR, Fésüs L (2006) Coeliac autoantibodies can enhance transamidating and inhibit GTPase activity of tissue transglutaminase: dependence on reaction environment and enzyme fitness. J Autoimmun 26:278–287
Király R, Csosz E, Kurtán T, Antus S, Szigeti K, Simon-Vecsei Z, Korponay-Szabó IR, Keresztessy Z, Fésüs L (2009) Functional significance of five noncanonical Ca2+-binding sites of human transglutaminase 2 characterized by site-directed mutagenesis. FEBS J 276:7083–7096
Kohler HP, Stickland MH, Ossei-Gerning N, Carter A, Mikkola H, Grant PJ (1998) Association of a common polymorphism in the factor XIII gene with myocardial infarction. Thromb Haemost 79:8–13
Laki K, Lóránd L (1948) On the solubility of fibrin clots. Science 108:280
Loewy AG (1968) Enzymatic control of insoluble fibrin solution. In: Laki K (ed) Fibrinogen. Marcel Dekker, New York, pp 185–224
Lorand L, Downey J, Gotoh T, Jacobsen A, Tokura S (1968) The transpeptidase system which crosslinks fibrin by gamma-glutamyle-episilon-lysine bonds. Biochem Biophys Res Commun 31:222–230
Matacić S, Loewy AG (1968) The identification of isopeptide crosslinks in insoluble fibrin. Biochem Biophys Res Commun 30:356–362
Muszbek L, Bereczky Z, Bagoly Z, Shemirani AH, Katona E (2010) Factor XIII and atherothrombotic diseases. Semin Thromb Hemost 36:18–33
Mycek MJ, Clarke DD, Neidle A, Waelsch H (1959) Amine incorporation into insulin as catalysed by transglutaminase. Arch Biochem Biophys 84:528–540
Ng PC, Henikoff S (2003) SIFT: predicting amino acid changes that affect protein function. Nucl Acids Res 31:3812–3814
Pisano JJ, Finlayson JS, Peyton MP (1968) Cross-link in fibrin polymerized by factor 13: epsilon-(gamma-glutamyl)lysine. Science 160:892–893
Porzio O, Massa O, Cunsolo V, Colombo C, Malaponti M, Bertuzzi F, Hansen T, Johansen A, Pedersen O, Meschi F, Terrinoni A, Melino G, Federici M, Decarlo N, Menicagli M, Campani D, Marchetti P, Ferdaoussi M, Froguel P, Federici G, Vaxillaire M, Barbetti F (2007) Missense mutations in the TGM2 gene encoding transglutaminase 2 are found in patients with early-onset type 2 diabetes. Hum Mutat 28:1150
Saha N, Aston CE, Low PS, Kamboh MI (2000) Racial and genetic determinants of plasma factor XIII activity. Genet Epidemiol 19:440–455
Sarkar NK, Clarke DD, Waelsch H (1957) An enzymically catalyzed incorporation of amines into proteins. Biochim Biophys Acta 25:451–452
Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE (2006) The consensus coding sequences of human breast and colorectal cancers. Science 314:268–274
Szondy Z, Sarang Z, Molnar P, Nemeth T, Piacentini M, Mastroberardino PG, Falasca L, Aeschlimann D, Kovacs J, Kiss I, Szegezdi E, Lakos G, Rajnavolgyi E, Birckbichler PJ, Melino G, Fesus L (2003) Transglutaminase 2−/− mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc Natl Acad Sci USA 100:7812–7817
The 1000 Genomes Project Consortium (2010) A map of human genome variation from population-scale sequencing. Nature 467:1061–1073
Thorisson GA, Smith AV, Krishnan L, Stein LD (2005) The International HapMap Project Web site. Genome Res 15:1591–1593
Trejo-Skalli AV, Velasco PT, Murthy SN, Lorand L, Goldman RD (1995) Association of a transglutaminase-related antigen with intermediate filaments. Proc Natl Acad Sci USA 92:8940–8944
Var A, Utük O, Akçali S, Sanlidağ T, Uyanik BS, Dinç G (2009) Impact of hemostatic gene single point mutations in patients with non-diabetic coronary artery disease. Mol Biol Rep 36:2235–2243
Acknowledgments
This work has been supported by grants from the Hungarian Scientific Research Fund (OTKA NI 67877), TAMOP-4.2.2-08/1/2008-0015 and TÁMOP 4.2.1./B-09/1/KONV-2010-0007 projects implemented through the New Hungary Development Plan, co-financed by the European Social Fund, an ETT grant from the Hungarian Ministry of Health, European Union MRTNCT-2006-036032 and MRTN-CT 2006-035624.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Király, R., Barta, E. & Fésüs, L. Polymorphism of transglutaminase 2: unusually low frequency of genomic variants with deficient functions. Amino Acids 44, 215–225 (2013). https://doi.org/10.1007/s00726-011-1194-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-1194-6