Abstract
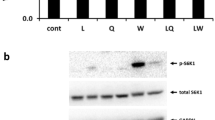
Leucine (LEU) is recognized as a major regulator of muscle protein synthesis (MPS). Citrulline (CIT) is emerging as a potent new regulator. The aim of our study was to compare MPS modulation by CIT and LEU in food-deprived rats and to determine whether their action was driven by similar mechanisms. Rats were either freely fed (F, n = 10) or food deprived for 18 h. Food-deprived rats were randomly assigned to one of four groups and received per os, i.e., gavage, saline (S, n = 10), l-leucine (1.35 g/kg, LEU, n = 10), l-citrulline (1.80 g/kg CIT, n = 10) or isonitrogenous non-essential amino acids (NEAA, n = 10). After gavage, the rats were injected with a flooding dose of [13C] valine to determine MPS. The rats were killed 50 min after the injection of the flooding dose. Blood was collected for amino acid, glucose and insulin determinations. Tibialis anterior muscles were excised for determination of MPS and for Western blot analyses of the PI3K/Akt, mTORC1, ERK1/2/MAPK pathways and AMP kinase component. MPS was depressed by 61% in starved rats (Saline vs. Fed, P < 0.05). Administration of amino acids (NEAA, LEU or CIT) completely abolished this decrease (NEAA, CIT, LEU vs. Fed, NS). Food deprivation affected the phosphorylation status of the mTORC1 pathway and AMP kinase (Saline vs. Fed, P < 0.05). LEU and CIT administration differently stimulated the mTORC1 pathway (LEU > CIT). LEU but not CIT increased the phosphorylation of rpS6 at serine 235/236. Our findings clearly demonstrated that both CIT and LEU were able to stimulate MPS, but this effect was likely related to the nitrogen load. LEU, CIT and NEAA may have different actions on MPS in this model as they share different mTORC1 regulation capacities.



Similar content being viewed by others
References
Anthony JC, Anthony TG, Layman DK (1999) Leucine supplementation enhances skeletal muscle recovery in rats following exercise. J Nutr 129:1102–1106
Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS (2000a) Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr 130:139–145
Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR (2000b) Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130:2413–2419
Anthony JC, Lang CH, Crozier SJ et al (2002a) Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab 282:E1092–E1101
Anthony JC, Reiter AK, Anthony TG et al (2002b) Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E-BP1 or S6K1 phosphorylation. Diabetes 51:928–936
Baum JI, OʼConnor JC, Seyler JE, Anthony TG, Freund GG, Layman DK (2005) Leucine reduces the duration of insulin-induced PI 3-kinase activity in rat skeletal muscle. Am J Physiol Endocrinol Metab 288:E86–E91
Chambon-Savanovitch C, Felgines C, Farges MC et al (1999) Comparative study of glycine, alanine or casein as inert nitrogen sources in endotoxemic rats. J Nutr 129:1866–1870
Chanseaume E, Giraudet C, Gryson C et al (2007) Enhanced muscle mixed and mitochondrial protein synthesis rates after a high-fat or high-sucrose diet. Obesity 15:853–859
Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS (2005) Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr 135:376–382
Dinckinson JM, Rasmussen B (2011) Essential amino acids sensing, signalling, and transport in the regulation of human muscle protein metabolism. Curr Opin Clin Nutr Metab Care 14:83–88
Duchêne S, Audoin E, Crochet S, duclos MJ, Dupont J, Tesseraud S (2008) Involvement of the ERK1/2 MAPK pathway in insulin-induced S6K1 activation in avian cells. Domest Anim Endocrinol 34:63–73
Fujita S, Dreyer HC, Drummond MJ et al (2007) Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol 582:813–823
Garlick PJ, Grant I (1988) Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched-chain amino acids. Biochem J 254:579–584
Gautsch TA, Anthony JC, Kimball SR, Paul GL, Layman DK, Jefferson LS (1998) Availability of eIF4E regulates skeletal muscle protein synthesis during recovery from exercise. Am J Physiol 274:C406–C414
Grimble RF, Jackson AA, Persaud C, Wride MJ, Delers F, Engler R (1992) Cysteine and glycine supplementation modulate the metabolic response to tumor necrosis factor α in rats fed a low protein diet. J Nutr 122:2066–2073
Hamel FG, Upward JL, Siford GL, Duckworth WC (2003) Inhibition of proteasome activity by selected amino acids. Metab Clin Exp 52:810–814
Hardie DG, Sakamoto K (2006) AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology 21:48–60
Ikejima K, Iimuro Y, Forman DT, Thurman RG (1996) A diet containing glycine improves survival in endotoxin shock in the rat. Am J Physiol 271:G97–G103
Kimball SR, Jefferson LS (2006) New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr 83:500S–507S
Le Plenier S, Walrand S, Cynober L, Moinard C (2011) Direct action of citrulline on muscle protein synthesis: role of the mTORC1 pathway. Clin Nutr 6(S1):20
Lee MY, Jo SD, Lee JH, Han HJ (2008) l-leucine increases [3H]-thymidine incorporation in chicken hepatocytes: involvement of PKC, PI3K/Akt, ERK1/2, and mTOR signaling pathways. J Cell Biochem 105:1410–1419
Moinard C, Cynober L (2007) Citrulline: a new player in the control of nitrogen homeostasis. J Nutr 137:1621S–1625S
Neveux N, David P, Cynober L (2004) Measurement of amino acid concentration in biological fluids and tissues using ion-exchange chromatography. In: Cynober L (ed) Metabolic and therapeutic aspects of amino acids in clinical nutrition, CRC Press, Boca Raton, pp 17–28
Nicklin P, Bergman P, Zhang B et al (2009) Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136:521–534
Osowska S, Duchemann T, Walrand S et al (2006) Citrulline modulates muscle protein metabolism in old malnourished rats. Am J Physiol Endocrinol Metab 291:E582–E586
Perez-Sala D, Parrilla R, Ayuso MS (1987) Key role of l-alanine in the control of hepatic protein synthesis. Biochem J 241:491–498
Prod’homme M, Rieu I, Balage M, Dardevet D, Grizard J (2004) Insulin and amino acids both strongly participate to the regulation of protein metabolism. Curr Opin Clin Nutr Metab Care 7:71–77
Roth E, Zellner M, Wessner B et al (2003) Glycine—an inert amino acid comes alive. Nutrition 19:817–818
Smith K, Reynolds N, Downie S, Patel A, Rennie MJ (1998) Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol 275:E73–E78
Stipanuk MH (2007) Leucine and protein synthesis: mTOR and beyond. Nutr Rev 65:122–129
Tardif N, Salles J, Landrier JF et al (2011) Oleate-enriched diet improves insulin sensitivity and restores muscle protein synthesis in old rats. Clin Nutr (Epub ahead of print)
Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR (2003) Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 78:250–258
Wilson GJ, Layman DK, Moulton CJ et al (2011) Leucine or carbohydrate supplementation reduces AMPK and eEF2 phosphorylation and extends postprandial muscle protein synthesis in rats. Am J Physiol Endocrinol Metab (Epub ahead of print)
Acknowledgments
We thank Sylviane Darquy for conducting the insulin and glucose assays, Julie Marc for her help in amino acid determinations, and Thomas Camus for animal care and management. We thank also Harvard Apparatus and Minerve for providing devices used in this study. This work was supported by a grant (EA 4466) from the French Ministry of Research and Technology under a 4-year contract.
Conflict of interest
S. Le Plénier, L. Cynober and C. Moinard are shareholders of Citrage Company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Le Plénier, S., Walrand, S., Noirt, R. et al. Effects of leucine and citrulline versus non-essential amino acids on muscle protein synthesis in fasted rat: a common activation pathway?. Amino Acids 43, 1171–1178 (2012). https://doi.org/10.1007/s00726-011-1172-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-1172-z