Abstract
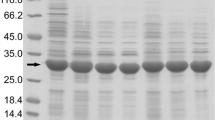
In order to produce recombinant microbial transglutaminase (rMTG) which is free of the activating protease, dispase was used to activate the pro-rMTG followed by immobilized metal affinity chromatography (IMAC). As shown by MALDI-MS, the dispase does not only cleave the pro-sequence, but unfortunately also cleaves within the C-terminal histidine-tag. Hence, the active rMTG cannot properly bind to the IMAC material. As an alternative, proteinase K was investigated. This protease was successfully applied for the activation of purified pro-rMTG either as free or immobilized enzyme and the free enzyme was also applicable directly in the crude cell extract of E. coli. Thus, it enables a simple two-step activation/purification procedure resulting in protease-free and almost pure transglutaminase preparations. The protocol has been successfully applied to both, wild-type transglutaminase of Streptomyces mobaraensis as well as to the highly active variant S2P. Proteinase K activates the pro-rMTG without unwanted degradation of the histidine-tag. It turned out to be very important to inhibit proteinase K activity, e.g., by PMSF, prior to protein separation by SDS–PAGE.






Similar content being viewed by others
Abbreviations
- BDM:
-
Bio dry mass
- DNA:
-
Deoxyribonucleic acid
- E. coli :
-
Escherichia coli
- HESylation:
-
Modification of proteins by hydroxy ethyl starch (HES)
- MALDI-MS:
-
Matrix assisted laser desorption ionization mass spectrometry
- His-tag:
-
Histidine-tag
- IMAC:
-
Immobilized metal affinity chromatography
- MTG:
-
Microbial transglutaminase of Streptomyces mobaraensis
- PMSF:
-
Phenylmethylsulfonyl fluoride
- pro-rMTG:
-
Inactive pro-enzyme of the recombinant microbial transglutaminase
- rMTG:
-
Recombinant microbial his-tagged transglutaminase (variant of Streptomyces mobaraensis TG)
- rMTG(S2P):
-
Recombinant microbial his-tagged transglutaminase (thermostable variant of Streptomyces mobaraensis TG)
- SDS–PAGE:
-
Sodium dodecylsulfate polyacrylamide gel electrophoresis
- TG:
-
Transglutaminase
References
Ando H, Adachi M, Umeda K, Matsuura A, Nonaka M, Uchio R, Tanaka H, Motoki M (1989) Purification and characteristics of a novel transglutaminase derived from microorganisms. Agric Biol Chem 53(10):2613–2617
Beninati S, Piacentini M (2004) The transglutaminase family: an overview: mini review article. Amino Acids 26(4):367–372
Besheer A, Hertel TC, Kressler J, Mäder K, Pietzsch M (2009) Enzymatically catalyzed HESylation using microbial transglutaminase: proof of feasibility. J Pharm Sci 98:4420–4428
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buettner K, Hertel TC, Pietzsch M (2011) Increased thermostability of microbial transglutaminase by combination of several hot spots evolved by random and saturation mutagenesis. Amino Acids. doi:10.1007/s00726-011-1015-y
Cardamone JM (2007) Enzyme-mediated crosslinking of wool. part I: transglutaminase. Text Res J 77(4):214–221
Chou S-Y (2009) Transglutaminase for crosslinking antigen to generate polyvalent antigens as vaccines against human pathogens and for antibody production. US Patent 7485438
Date M, Yokoyama K, Umezawa Y, Matsui H, Kikuchi Y (2003) Production of native-type Streptoverticillium mobaraense transglutaminase in Corynebacterium glutamicum. Appl Environ Microbiol 69(5):3011–3014
Ebeling W, Hennrich N, Klockow M, Metz H, Orth HD, Lang H (1974) Proteinase K from Tritirachium album Limber. Eur J Biochem 47(1):91–97
Folk JE (1969) Mechanism of action of guinea pig liver transglutaminase. VI. Order of substrate addition. J Biol Chem 244(13):3707–3713
Gross-Bellard M, Oudet P, Chambon P (1973) Isolation of high-molecular-weight DNA from mammalian cells. Eur J biochem 36(1):32–38
Itaya H, Kikuchi Y (2008) Secretion of Streptomyces mobaraensis pro-transglutaminase by Coryneform bacteria. Appl Microbiol Biotechnol 78(4):621–625
Kamata Y, Ishikawa E, Motoki M (1992) Enzyme immobilization on ion exchangers by forming an enzyme coating with transglutaminase as a crosslinker. Biosci Biotechnol Biochem 56(8):1323–1324
Kikuchi Y, Date M, Yokoyama K, Umezawa Y, Matsui H (2003) Secretion of active-form Streptoverticillium mobaraense transglutaminase by Corynebacterium glutamicum: processing of the pro-transglutaminase by a cosecreted subtilisin-like protease from Streptomyces albogriseolus. Appl Environ Microbiol 69(1):358–366
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(259):680–685
Mariniello L, Porta R (2005) Transglutaminases as biotechnological tools. Prog Exp Tumor Res 38:174–191
Marx CK, Hertel TC, Pietzsch M (2008a) Purification and activation of a recombinant histidine-tagged pro-transglutaminase after soluble expression in E. coli and characterization of the active enzyme. Enzym Microb Technol 42:568–575
Marx CK, Hertel TC, Pietzsch M (2008b) Random mutagenesis of a recombinant microbial transglutaminase for the generation of thermostable and heat sensitive variants. J Biotechnol 136:156–162
Pasternack R, Dorsch S, Otterbach JT, Robenek IR, Wolf S, Fuchsbauer HL (1998) Bacterial pro-transglutaminase from Streptoverticillium mobaraense—purification, characterisation and sequence of the zymogen. Eur J Biochem 257(3):570–576
Patzsch K, Riedel K, Pietzsch M (2010) Parameter optimization for protein film production using microbial transglutaminase. Biomacromolecules 11:896–903
Portilla-Rivera OM, Tellez-Luis SJ, Ramirez de Leon JA, Vazquez M (2009) Production of microbial transglutaminase on media made from sugar cane molasses and glycerol. Food Technol Biotechnol 47(1):19–26
Russell MW, Bergmeier LA, Zanders ED, Lehner T (1980) Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun 28(2):486–493
Sato H (2002) Enzymatic procedure for site-specific pegylation of proteins. Adv Drug Deliv Rev 54(4):487–504
Sommer C, Volk N, Pietzsch M (2011) Model based optimization of the fed-batch production of a highly active transglutaminase variant in E. coli. Protein Expr Purif 77:9–19
Synowiecki J, Wolosowska S (2006) Immobilization of thermostable β-glucosidase from Sulfolobus shibatae by crosslinking with transglutaminase. Enzyme Microb Technol 39(7):1417–1422
Yang H-L, Pan L, Lin Y (2009) Purification and on-column activation of a recombinant histidine-tagged pro-transglutaminase after soluble expression in Escherichia coli. Biosci Biotechnol Biochem 73(11):2531–2534
Zotzel J, Keller P, Fuchsbauer HL (2003) Transglutaminase from Streptomyces mobaraensis is activated by an endogenous metalloprotease. Eur J Biochem 270(15):3214–3222
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sommer, C., Hertel, T.C., Schmelzer, C.E.H. et al. Investigations on the activation of recombinant microbial pro-transglutaminase: in contrast to proteinase K, dispase removes the histidine-tag. Amino Acids 42, 997–1006 (2012). https://doi.org/10.1007/s00726-011-1016-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-1016-x