Abstract
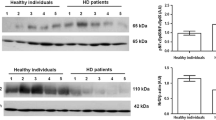
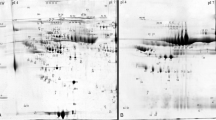
We analyzed proteomic profiles in monocytes of chronic kidney disease (CKD) patients and healthy control subjects. Two-dimensional electrophoresis (2-DE) and silver staining indicated differences in protein pattern. Among the analyzed proteins, superoxide dismutase type 1 (SOD1), which was identified both by MS/MS mass-spectrometry and immunoblotting, was reduced in kidney disease. We characterized SOD1 protein amount, using quantitative in-cell Western assay and immunostaining of 2-DE gel blots, and SOD1 gene expression, using quantitative real-time polymerase chain reaction (PCR), in 98 chronic hemodialysis (HD) and 211 CKD patients, and 34 control subjects. Furthermore, we showed that different SOD1 protein species exist in human monocytes. SOD1 protein amount was significantly lower in HD (normalized SOD1 protein, 27.2 ± 2.8) compared to CKD patients (34.3 ± 2.8), or control subjects (48.0 ± 8.6; mean ± SEM; P < 0.05). Analysis of SOD1 immunostaining showed significantly more SOD1 protein in control subjects compared to patients with CKD or HD (P < 0.0001, analysis of main immunoreactive protein spot). SOD1 gene expression was significantly higher in HD (normalized SOD1 gene expression, 17.8 ± 2.3) compared to CKD patients (9.0 ± 0.7), or control subjects (5.5 ± 1.0; P < 0.0001). An increased SOD1 gene expression may indicate increased protein degradation in patients with CKD and compensatory increase of SOD1 gene expression. Taken together, we show reduced SOD1 protein amount in monocytes of CKD, most pronounced in HD patients, accompanied by increased SOD1 gene expression.






Similar content being viewed by others
References
Berggren K, Steinberg TH, Lauber WM, Carroll JA, Lopez MF, Chernokalskaya E, Zieske L, Diwu Z, Haugland RP, Patton WF (1999) A luminescent ruthenium complex for ultrasensitive detection of proteins immobilized on membrane supports. Anal Biochem 276:129–143
Bumann D, Meyer TF, Jungblut PR (2001) Proteome analysis of the common human pathogen helicobacter pylori. Proteomics 1:473–479
Choi J, Rees HD, Weintraub ST, Levey AI, Chin LS, Li L (2005) Oxidative modifications and aggregation of Cu/Zn superoxide dismutase associated with Alzheimer’s and Parkinson’s diseases. J Biol Chem 280:11648–11655
Didion SP, Kinzenbaw DA, Schrader LI, Faraci FM (2006) Heterozygous CuZn superoxide dismutase deficiency produces a vascular phenotype with aging. Hypertension 48:1072–1079
Diedrich M, Tadic J, Mao L, Wacker MA, Nebrich G, Hetzer R, Regitz-Zagrosek V, Klose J (2007) Heart protein expression related to age and sex in mice and humans. Int J Mol Med 20:865–874
Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang TT (2005) CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene 24:367–380
Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM (2002) The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 62:1524–1538
Hoehenwarter W, Ackermann R, Zimny-Arndt U, Kumar NM, Jungblut PR (2006a) The necessity of functional proteomics: protein species and molecular function elucidation exemplified by in vivo alpha A crystallin N-terminal truncation. Amino Acids 31:317–323
Hoehenwarter W, Klose J, Jungblut PR (2006b) Eye lens proteomics. Amino Acids 30:369–389
Jabeen R, Mohammad AA, Elefano EC, Petersen JR, Saleemuddin M (2006) Antibodies and Fab fragments protect Cu, Zn-SOD against methylglyoxal-inducd inactivation. Biochim Biophys Acta 1760:1167–1174
Jabeen R, Saleemuddin M, Petersen J, Mohammad A (2007) Inactivation and modification of superoxide dismutase by glyoxal: prevention by antibodies. Biochimie 89:311–318
Jungblut PR, Holzhütter HG, Apweiler R, Schlüter H (2008) The speciation of the proteome. Chem Cent J 2:16
Klose J, Kobalz U (1995) Two-dimensional electrophoresis of proteins: an updated protocol and implications for a functional analysis of the genome. Electrophoresis 16:1034–1059
Knepper MA (2002) Proteomics and the kidney. J Am Soc Nephrol 13:1398–1408
Krueger K, Koch K, Jühling A, Tepel M, Scholze A (2010) Low expression of thiosulfate sulfurtransferase (rhodanese) predicts mortality in hemodialysis patients. Clin Biochem 43:95–101
Liu D, Scholze A, Zhu Z, Krueger K et al (2006) Transient receptor potential channels in essential hypertension. J Hypertens 24:1105–1114
Locatelli F, Canaud B, Eckardt K, Stenvinkel P et al (2003) Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant 18:1272–1280
Merino A, Nogueras S, Buendía P, Ojeda R, Carracedo J, Ramirez-Chamond R, Martin-Malo A, Aljama P (2008) Microinflammation and endothelial damage in hemodialysis. Contrib Nephrol 161:83–88
Monteleone G, Del Vecchio Blanco G, Monteleone I, Fina D, Caruso R, Gioia V, Ballerini S, Federici G, Bernardini S, Pallone F, MacDonald TT (2005) Post-transcriptional regulation of Smad7 in the gut of patients with inflammatory bowel disease. Gastroenterology 129:1420–1429
Nguyen T, Sherratt PJ, Pickett CB (2003) Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 43:233–260
Nolan CR (2005) Strategies for improving long-term survival in patients with ESRD. J Am Soc Nephrol 16(Suppl 2):S120–S127
Park EY, Rho HM (2002) The transcriptional activation of the human copper/zinc superoxide dismutase gene by 2,3,7,8-tetrachlorodibenzo-p-dioxin through two different regulator sites, the antioxidant responsive element and xenobiotic responsive element. Moll Cell Biochem 240:47–55
Quehenberger O (2005) Molecular mechanisms regulating monocyte recruitment in atherosclerosis. J Lipid Res 46:1582–1590
Roberts MA, Hare DL, Ratnaike S, Ierino FL (2006) Cardiovascular biomarkers in CKD: pathophysiology and implications for clinical management of cardiac disease. Am J Kidney Dis 48:341–360
Santibanez JF, Letamendia A, Perez-Barriocanal F, Silvestri C, Saura M, Vary CP, Lopez-Novoa JM, Attisano L, Bernabeu C (2007) Endoglin increases eNOS expression by modulating Smad2 protein levels and Smad2-dependent TGF-beta signalin. J Cell Physiol 210:456–468
Sasse J, Gallagher SR (2008) Detection of proteins on blot transfer membranes. Curr Protoc Immunol 83:8.10B.1–8.10B.6
Scheler C, Lamer S, Pan Z, Li XP, Salnikow J, Jungblut P (1998) Peptide mass fingerprint sequence coverage from differently stained proteins on two-dimensional electrophoresis patterns by matrix assisted laser desorption/ionization-mass spectrometry (MALDI-MS). Electrophoresis 19:918–927
Tan R, Zhang J, Tan X, Zhang X, Yang J, Liu Y (2006) Downregulation of SnoN expression in obstructive nephropathy is mediated by an enhanced ubiquitin-dependent degradation. J Am Soc Nephrol 17:2781–2791
Tepel M, van der Giet M, Statz M, Jankowski J, Zidek W (2003) The antioxidant, acetylcysteine, reduces cardiovascular events in patients with endstage renal failure—a randomized controlled trial. Circulation 107:992–995
Thilo F, Scholze A, Liu DY, Zidek W, Tepel M (2008) Association of transient receptor potential canonical type 3 (TRPC3) channel transcripts with proinflammatory cytokines. Arch Biochem Biophys 471:57–62
Vanholder R, Baurmeister U, Brunet P, Cohen G, Glorieux G, Jankowski J, European Uremic Toxin Work Group (2008) A bench to bedside view of uremic toxins. J Am Soc Nephrol 19:863–870
Vaziri ND (2004) Oxidative stress in uremia: nature, mechanisms, and potential consequences. Semin Nephrol 24:469–473
Watanabe T, Yasunari K, Nakamura M, Maeda K (2006) Carotid artery intima-media thickness and reactive oxygen species formation by monocytes in hypertensive patients. J Human Hypertens 20:336–340
Wittmann-Liebold B, Graack HR, Pohl T (2006) Two-dimensional gel electrophoresis as tool for proteomics studies in combination with protein identification by mass spectrometry. Proteomics 6:4688–4703
Yilmaz MI, Saglam M, Caglar K, Cakir E et al (2005) The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis 47:42–53
Zhao Y, Jensen ON (2009) Modification-specific proteomics: strategies for characterization of post-translational modifications using enrichment techniques. Proteomics 9:4632–4641
Zwolinska D, Grzeszczak W, Szczepanska M, Kilis-Pstrusinska K, Szprynger K (2006) Lipid peroxidation and antioxidant enzymes in children on maintenance dialysis. Pediatr Nephrol 21:705–710
Acknowledgments
This study was supported by Dr. Werner Jackstädt-Stiftung, Wuppertal, and Sonnenfeld-Stiftung, Berlin, Germany.
Conflict of interest
All authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scholze, A., Krueger, K., Diedrich, M. et al. Superoxide dismutase type 1 in monocytes of chronic kidney disease patients. Amino Acids 41, 427–438 (2011). https://doi.org/10.1007/s00726-010-0763-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0763-4