Abstract
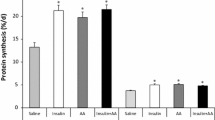
Leucine is unique among the amino acids in its ability to promote protein synthesis by activating translation initiation via the mammalian target of rapamycin (mTOR) pathway. Previously, we showed that leucine infusion acutely stimulates protein synthesis in fast-twitch glycolytic muscle of neonatal pigs but this response cannot be maintained unless the leucine-induced fall in amino acids is prevented. To determine whether leucine can stimulate protein synthesis in muscles of different fiber types and in visceral tissues of the neonate in the long-term if baseline amino acid concentrations are maintained, overnight fasted neonatal pigs were infused for 24 h with saline, leucine (400 μmol kg−1 h−1), or leucine with replacement amino acids to prevent the leucine-induced hypoaminoacidemia. Changes in the fractional rate of protein synthesis and activation of mTOR, as determined by eukaryotic initiation factor 4E binding protein (4E-BP1) and S6 kinase 1 (S6K1) phosphorylation, in the gastrocnemius and masseter muscles, heart, liver, jejunum, kidney, and pancreas were measured. Leucine increased mTOR activation in the gastrocnemius and masseter muscles, liver, and pancreas, in both the absence and presence of amino acid replacement. However, protein synthesis in these tissues was increased only when amino acids were infused to maintain baseline levels. There were no changes in mTOR signaling or protein synthesis in the other tissues we examined. Thus, long-term infusion of leucine stimulates mTOR signaling in skeletal muscle and some visceral tissues but the leucine-induced stimulation of protein synthesis in these tissues requires sustained amino acid availability.



Similar content being viewed by others
References
Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR (2000) Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130:2413–2419
Anthony JC, Anthony TG, Kimball SR, Jefferson LS (2001) Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr 131:856S–860S
Anthony JC, Reiter AK, Anthony TG, Crozier SJ, Lang CH, MacLean DA, Kimball SR, Jefferson LS (2002) Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E-BP1 or S6K1 phosphorylation. Diabetes 51:928–936
Ban H, Shigemitsu K, Yamastsuji T, Haisa M, Nakajo T, Takaoka M, Nobuhisa T, Gunduz M, Tanaka N, Naomota Y (2004) Arginine and leucine regulate p70 S6 kinase and 4E-BP1 in intestinal epithelial cells. Int J Mol Med 13:537–543
Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ, McAvoy S (1995) Nutrient-independent and nutrient-dependent factors stimulate protein synthesis in colostrum-fed newborn pigs. Pediatr Res 37:593–599
Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS (2005) Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr 135:376–382
Davis TA, Fiorotto MF (2009) Regulation of muscle growth in neonates. Curr Opin Clin Nutr 12:78–85
Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ (1989) Protein turnover in skeletal muscle of sucking rats. Am J Physiol 257:R1141–R1146
Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ (1993) Enhanced response of muscle protein synthesis and plasma insulin to food intake in suckled rats. Am J Physiol 265:R334–R340
Davis TA, Burrin DG, Fiorotto ML, Nguyen HV (1996) Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than in 26-day-old pigs. Am J Physiol Endocrinol Metab 33:E802–E809
Davis TA, Fiorotto ML, Nguyen HV, Burrin DG (1999) Aminoacyl-tRNA and tissue free amino acid pools are equilibrated after a flooding dose of phenylalanine. Am J Physiol Endocrinol Metab 277:E103–E109
Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR (2000) Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab 279:E1226–E1234
Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O’Connor PMJ (2002) Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab 282:E880–E890
Ehrenkranz RA (2007) Early, aggressive nutritional management for very low birth weight infants: what is the evidence? Semin Perinatol 31:48–55
Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA (2005) Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab 288:E914–E921
Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA (2006) Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab 290:E612–E621
Escobar J, Frank JW, Suryawan A, Nguyen HV, Davis TA (2007) Amino acid availability and age affect the leucine stimulation of protein synthesis and eIF4F formation in muscle. Am J Physiol Endocrinol Metab 293:E1615–E1621
Frank JW, Escobar J, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA (2006) Dietary protein and lactose increase translation initiation factor activation and tissue protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab 290:E225–E233
Garlick PJ, Mcnurlan MA, Preedy VR (1980) A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J 192:719–723
Gingras AC, Raught B, Sonenberg N (2001) Regulation of translation initiation by FRAP/mTOR. Genes Dev 15:807–826
Hagenfeldt L, Eriksson S, Wahren J (1980) Influence of leucine on arterial concentrations and regional exchange of amino acids in healthy subjects. Clin Sci (Lond) 59:173–181
Kimball SR, Jefferson LS (2004) Regulation of global and specific mRNA translation by oral administration of branched-chain amino acids. Biochem Biophys Res Commun 313:423–427
Koopman R, Verdijk LB, Beelen M, Gorselink M, Kruseman AN, Wagenmakers AJM, Kuipers H, van Loon LJC (2008) Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Br J Nutr 99:571–580
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lynch CJ, Hutson SM, Patson BJ, Vaval A, Vary TC (2002a) Tissue-specific effects of chronic dietary leucine and norleucine supplementation on protein synthesis in rats. Am J Physiol Endocrinol Metab 283:E824–E835
Lynch CJ, Patson BJ, Anthony JC, Vaval A, Jefferson LS, Vary TC (2002b) Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am J Physiol Endocrinol Metab 283:E503–E513
Morgan HE, Beinlich CJ (1997) Contributions of increased efficiency and capacity of protein synthesis to rapid cardiac growth. Mol Cell Biochem 176:145–151
Munro HN, Fleck A (1969) Analysis of tissues and body fluids for nitrogenous constituents. In: Munro HN (ed) Mammalian protein metabolism, vol 3. Academic Press, New York, pp 465–483
Nair KS, Matthews DE, Welle SL, Braiman T (1992) Effect of leucine on amino acid and glucose metabolism in humans. Metabolism 41:643–648
Norton LE, Layman DK, Bunpo P, Anthony TG, Brana DV, Garlick PJ (2009) The leucine content of a complete meal directs peak activation but not duration of skeletal muscle protein synthesis and mammalian target of rapamycin signaling in rats. J Nutr 139:1103–1109
O’Connor PMJ, Bush JA, Suryawan A, Nguyen HV, Davis TA (2003a) Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab 284:E110–E119
O’Connor PMJ, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA (2003b) Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab 285:E40–E53
O’Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA (2004) Regulation of neonatal liver protein synthesis by insulin and amino acids in pigs. Am J Physiol Endocrinol Metab 286:E994–E1003
Reiter AK, Anthony TG, Anthony JC, Jefferson LS, Kimball SR (2004) The mTOR signaling pathway mediates control of ribosomal protein mRNA translation in rat liver. Int J Biochem Cell Biol 36:2169–2179
Rhodes JM, Liu Y, Niu X, Surendran S, Wu G (2008) Arginine stimulates cdx2-transformed intestinal epithelial cell migration via a mechanism requiring both nitric oxide and phosphorylation of p70 S6 kinase. J Nutr 138:1652–1657
Saigal S, Stoskopf BL, Streiner DL, Burrows E (2001) Physical growth and current health status of infants who were of extremely low birth weight and controls at adolescence. Pediatrics 108:407–415
Sans MD, Tashiro M, Vogel NL, Kimball SR, D’alecy LG, Williams JA (2006) Leucine activates pancreatic translational machinery in rats and mice through mTOR independently of CCK and insulin. J Nutr 136:1792–1799
Stipanuk MH (2007) Leucine and protein synthesis: mTOR and beyond. Nutr Rev 65:122–129
Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA (2008) Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab 295:868–875
Suryawan A, O’Connor PMJ, Bush JA, Nguyen HV, Davis TA (2009) Differential regulation of protein synthesis by amino acids and insulin in peripheral and visceral tissues of neonatal pigs. Amino Acids 37:97–104
Vary TC (2009) Oral leucine enhances myocardial protein synthesis in rats acutely administered ethanol. J Nutr 139:1439–1444
Wilson FA, Suryawan A, Gazzaneo MC, Orellana RA, Nguyen HV, Davis TA (2010) Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J Nutr 140:264–270
Xu G, Kwon G, Marshall CA, Lin T, Lawrence JC, McDaniel MJ (1998) Branched-chain amino acids are essential in the regulation of PHAS-I and p70 S6 kinase by pancreatic B-cells. J Biol Chem 273:28178–28184
Xu G, Kwon G, Cruz WS, Marshall CA, McDaniel MJ (2001) Metabolic regulation by leucine of translation initiation through the mTOR-signaling pathway by pancreatic B-cells. Diabetes 50:353–360
Acknowledgments
We thank Marta Fiorotto for helpful discussions, Jerome Stubblefield for care of animals, E. O’Brian Smith for statistical assistance, Adam Gillum for graphics and Linda Weiser for secretarial assistance. Funding for this research was received from the Ajinomoto Amino Acid Research Program, NIH R01 AR-44474, NIH K08 AR051563, and USDA/ARS Cooperative Agreement no. 58-6250-6-001. This work is a publication of the United States Department of Agriculture, Agricultural Research Service (USDA/ARS) Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilson, F.A., Suryawan, A., Orellana, R.A. et al. Differential effects of long-term leucine infusion on tissue protein synthesis in neonatal pigs. Amino Acids 40, 157–165 (2011). https://doi.org/10.1007/s00726-010-0629-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0629-9