Abstract
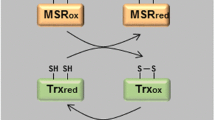
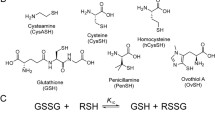
Selenocysteine is present in a variety of proteins and catalyzes the oxidation of thiols to disulfides and the reduction of disulfides to thiols. Here, we compare the kinetic and thermodynamic properties of cysteine with its selenium-containing analogon, selenocysteine. Reactions of simple selenols at pH 7 are up to four orders of magnitude faster than their sulfur analogs, depending on reaction type. In redox-related proteins, the use of selenium instead of sulfur can be used to tune electrode, or redox, potentials. Selenocysteine could also have a protective effect in proteins because its one-electron oxidized product, the selanyl radical, is not oxidizing enough to modify or destroy proteins, whereas the cysteine-thiyl radical can do this very rapidly.

Similar content being viewed by others
References
Akhlag MS, von Sonntag C (1987) Intermolecular H-abstraction of thiyl radicals from thiols and the intramolecular complexing of the thiyl radical with the thiol group in 1, 4-dithiothreitol: a pulse radiolysis study. Z Naturforsch 42c:134–140
Bachrach SM (1990) The group equivalent reaction. J Chem Educ 67:907–908
Besse D, Siedler F, Diercks T, Kessler H, Moroder L (1997) The redox potential of selenocystine in unconstrained cyclic peptides. Angew Chem Int Edit 36:883–885
Chaudière J, Courtin O, Leclaire J (1992) Glutathione oxidase activity of selenocysteine. Arch Biochem Biophys 296:328–336
Cleland WW (1964) Dithiothreitol: a new protective reagent for SH groups. Biochemistry 3:480–482
Flohé L, Günzler WA, Schock HH (1973) Glutathione peroxidase: a selenoenzyme. FEBS Lett 32:132–134
Frisch MJ et al (2003) Gaussian 03, Revision D.01
Gromer S et al (2003) Acitve sites of thioredoxin reductases: why selenoproteins? Proc Natl Acad Sci USA 100:12618–12623
Huber RE, Criddle RS (1967) Comparison of the chemical properties of selenocysteine and selenocystine with their sulfur analogs. Arch Biochem Biophys 122:164–173
Kanzok SM et al (2001) Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science 291:643–646
Lacey BM, Hondal RJ (2006) Characterization of mitochondrial thioredoxin reductase from C. elegans. Biochem Biophys Res Commun 346:629–636
Metanis N, Keinan E, Dawson PE (2006) Synthetic seleno-glutaredoxin 3 analogues are highly reducing oxidoreductases with enhanced catalytic efficiency. J Am Chem Soc 128:16684–16691
Nauser T, Pelling J, Schöneich C (2004) Thiyl radical reaction with amino acid side chains: rate constants for hydrogen transfer and relevance for posttranslational protein modification. Chem Res Toxicol 17:1323–1328
Nauser T, Dockheer S, Kissner R, Koppenol WH (2006) Catalysis of electron transfer by selenocysteine. Biochemistry 45:6038–6043
Nauser T, Casi G, Koppenol WH, Schöneich C (2008) Reversible intramolecular hydrogen transfer between cysteine thiyl radicals and glycine and alanine in model peptides: absolute rate constants derived from pulse radiolysis and laser flash photolysis. J Phys Chem B 112:15034–15044
Peskin AV, Low FM, Paton LN, Maghzal GJ, Hampton MB, Winterbourn CC (2007) The high reactivity of peroxiredoxin 2 with H2O2 is not reflected in its reaction with other oxidants and thiol reagents. J Biol Chem 282:11885–11892
Pleasants JC, Guo W, Rabenstein DL (1989) A comparative study of the kinetics of selenol/diselenide and thiol/disulfide exchange reactions. J Am Chem Soc 111:6553–6558
Rocher C, Lalanne J-L, Chaudière J (1992) Purification and properties of a recombinant sulfur analog of murin selenium-glutathione peroxidase. Eur J Biochem 205:955–960
Singh R, Whitesides GM (1991) Selenols catalyze the interchange reactions of dithiols and disulfides in water. J Org Chem 56:6931–6933
Winterbourn CC (1993) Superoxide as an intracellular radical sink. Free Radical Biol Med 14:85–90
Acknowledgments
We thank G. Grassi (Laboratorium für physikalische Chemie, ETH Zürich) for help with the synthesis of SeTT and DSeT. We thank ETH for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nauser, T., Steinmann, D. & Koppenol, W.H. Why do proteins use selenocysteine instead of cysteine?. Amino Acids 42, 39–44 (2012). https://doi.org/10.1007/s00726-010-0602-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0602-7