Abstract
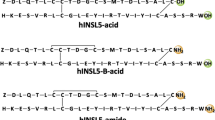
Insulin-like peptide 5 (INSL5) is a recently identified insulin superfamily member. Although it binds to and activates the G-protein coupled receptor, RXFP4, its precise biological function remains unknown. To help determine its function, significant quantities of INSL5 are required. In the present work, three single-chain INSL5 precursors were designed, two of which were successfully expressed in E. coli cells. The expressed precursors were solubilized from inclusion bodies, purified almost to homogeneity by immobilized metal-ion affinity chromatography, and then refolded in vitro. One precursor could be converted to two-chain human INSL5 bearing an extended N-terminus of the A-chain (designated long-INSL5) by sequential Lys-C endoproteinase and carboxypeptidase B treatment. The 6 residue A-chain N-terminal extension of long-INSL5 was subsequently removed by Aeromonas aminopeptidase to yield native INSL5 that was designated short-INSL5. Circular dichroism spectroscopic analysis and peptide mapping showed that the recombinant INSL5s adopted an insulin-like conformation and possessed the expected characteristic insulin-like disulfide linkages. Activity assay showed that both long- and short-INSL5 had full RXFP4 receptor activity compared with chemically synthesized human INSL5. This suggested that extension of the N-terminus of the A-chain of long-INSL5 did not adversely impact upon the binding to or activation of the RXFP4 receptor. However, the single-chain INSL5 precursor was inactive which indicated that a free C-terminus of the B-chain is critical for the activity of INSL5. Our present work thus provides an efficient approach for preparation of INSL5 and its analogs through recombinant expression in E. coli cells.







Similar content being viewed by others
Abbreviations
- CD:
-
Circular dichroism
- DTT:
-
Dithiothreitol
- GSSG:
-
Oxidized glutathione
- INSL5:
-
Insulin-like peptide 5
- IPTG:
-
Isopropyl β-D-thiogalactoside
- TCEP:
-
Tris(2-carboxyethyl)phosphine
- TFA:
-
Trifluoroacetic acid
- UV:
-
Ultra violet
References
Conklin D, Lofton-Day CE, Haldeman BA, Ching A, Whitmore TE, Lok S, Jaspers S (1999) Identification of INSL5, a new member of the insulin superfamily. Genomics 60:50–56
Haugaard-Jönsson LM, Hossain MA, Daly NL, Bathgate RA, Wade JD, Craik DJ, Rosengren KJ (2008) Structure of the R3/I5 chimeric relaxin peptide, a selective GPCR135 and GPCR142 agonist. J Biol Chem 283:23811–23828
Haugaard-Jönsson LM, Hossain MA, Daly NL, Craik DJ, Wade JD, Rosengren KJ (2009) Structure of human insulin-like peptide 5 and characterization of conserved hydrogen bonds and electrostatic interactions within the relaxin framework. Biochem J 419:619–627
Hossain MA, Bathgate RA, Kong CK, Shabanpoor F, Zhang S, Haugaard-Jönsson LM, Rosengren KJ, Tregear GW, Wade JD (2008a) Synthesis, conformation, and activity of human insulin-like peptide 5 (INSL5). ChemBioChem 9:1816–1822
Hossain MA, Rosengren KJ, Haugaard-Jonsson LM, Zhang S, Layfield S, Ferraro T, Daly NL, Tregear GW, Wade JD, Bathgate RA (2008b) The A-chain of human relaxin family peptides has distinct roles in the binding and activation of the different relaxin family peptide receptors. J Biol Chem 283:17287–17297
Hsu SY (1999) Cloning of two novel mammalian paralogs of relaxin/insulin family proteins and their expression in testis and kidney. Mol Endocrinol 13:2163–2174
Kuei C, Sutton S, Bonaventure P, Pudiak C, Shelton J, Zhu J, Nepomuceno D, Wu J, Chen J, Kamme F, Seierstad M, Hack MD, Bathgate RA, Hossain MA, Wade JD, Atack J, Lovenberg TW, Liu C (2007) R3(BDelta23 27)R/I5 chimeric peptide, a selective antagonist for GPCR135 and GPCR142 over relaxin receptor LGR7: in vitro and in vivo characterization. J Biol Chem 282:25425–25435
Lange C, Rudolph R (2009) Suppression of protein aggregation by L-arginine. Curr Pharm Biotechnol 10:408–414
Liu C, Chen J, Sutton S, Roland B, Kuei C, Farmer N, Sillard R, Lovenberg TW (2003) Identification of relaxin-3/INSL7 as a ligand for GPCR142. J Biol Chem 278:50765–50770
Liu C, Chen J, Kuei C, Sutton S, Nepomuceno D, Bonaventure P, Lovenberg TW (2005a) Relaxin-3/insulin-like peptide 5 chimeric peptide, a selective ligand for G protein-coupled receptor (GPCR)135 and GPCR142 over leucine-rich repeat-containing G protein-coupled receptor 7. Mol Pharmacol 67:231–240
Liu C, Kuei C, Sutton S, Chen J, Bonaventure P, Wu J, Nepomuceno D, Kamme F, Tran DT, Zhu J, Wilkinson T, Bathgate R, Eriste E, Sillard R, Lovenberg TW (2005b) INSL5 is a high affinity specific agonist for GPCR142 (GPR100). J Biol Chem 280:292–300
Nakakido M, Kudou M, Arakawa T, Tsumoto K (2009) To be excluded or to bind, that is the question: arginine effects on proteins. Curr Pharm Biotechnol 10:415–420
Shabanpoor F, Separovic F, Wade JD (2009) The human insulin superfamily of polypeptide hormones. Vitam Horm 80:1–31
Shirneshan K (2005) Expression, functional and regulation analysis of selected genes from the insulin family. Ph. D. dissertation, University of Gottingen (Germany), http://webdoc.sub.gwdg.de/diss/2006/shirneshan/
Tsumoto K, Umetsu M, Kumagai I, Ejima D, Philo JS, Arakawa T (2004) Role of arginine in protein refolding, solubilization, and purification. Biotechnol Prog 20:1301–1308
Zhu J, Kuei C, Sutton S, Kamme F, Yu J, Bonaventure P, Atack J, Lovenberg TW, Liu C (2008) Identification of the domains in RXFP4 (GPCR142) responsible for the high affinity binding and agonistic activity of INSL5 at RXFP4 compared to RXFP3 (GPCR135). Eur J Pharmacol 590:43–52
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program, no. 2010CB912604) and by the National Natural Science Foundation of China (30970609, 30700124). The studies at the HFI (Melbourne) were supported in part by NHMRC project grants (# 508995 and 509048) to JDW and RADB. We thank Linda Chan (FNI) for the amino acid analyses and Sharon Layfield (FNI) for the bioassays.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Luo, X., Bathgate, R.A.D., Zhang, WJ. et al. Design and recombinant expression of insulin-like peptide 5 precursors and the preparation of mature human INSL5. Amino Acids 39, 1343–1352 (2010). https://doi.org/10.1007/s00726-010-0586-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0586-3