Abstract
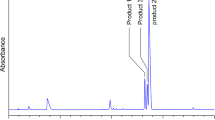
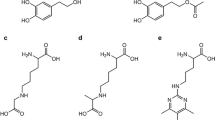
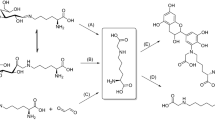
Pyridoxamine (PM) has long been known to inhibit protein glycation via various mechanisms of action. One such mechanism involves the scavenging of carbonyl compounds with glycating ability. Despite the abundant literature on this topic, few quantitative kinetic studies on the processes involved have been reported. In this work, we conducted a comparative kinetic study under physiological pH and temperature conditions of the reactions of PM, Ac-Phe-Lys and Ac-Cys with various glycating carbonyl compounds (viz. aldehydes, α-oxoaldehydes and ketones). The microscopic formation rate constants for Schiff bases of PM and various carbonyl compounds, k 1, are of the same order of magnitude as those for the Schiff bases of Ac-Phe-Lys. However, because PM exhibits a higher proportion of reactive form at physiological pH, its observed second-order rate constant is ca. five times greater than that for Ac-Phe-Lys. That could explain PM ability to compete with amino residues in protein glycation. On the other hand, the observed formation rate constant for thiohemiacetals is four orders of magnitude greater than the formation constants for the Schiff bases of PM, which excludes PM as a competitive inhibitor of Cys residues in protein glycation.








Similar content being viewed by others
References
Adrover M, Vilanova B, Muñoz F, Donoso J (2007) Pyridoxamine, a scavenger agent of carbohydrates. Int J Chem Kinet 39:154–167
Adrover M, Vilanova B, Frau J, Muñoz F, Donoso J (2008) The pyridoxamine action on Amadori compounds: a re-examination of its scavenging capacity and chelating effect. Bioorg Med Chem. doi: 10.1016/j.bmc.2008.04.002
Alderson NL, Wang Y, Blatnik M, Frizzell N, Walla MD, Lyons TJ, Alt N, Carson JA, Nagai R, Thorpe SR, Baynes JW (2006) S-(2-Succinyl)cysteine: A novel chemical modification of tissue proteins by a Krebs cycle intermediate. Arch Biochem Biophys 450:1–8
Aldini G, Dalle-Done I, Maffei-Facino R, Milzani A, Carini M (2007) Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med Res Rev 27:817–868
Amarnath V, Amarnath K, Amarnath K, Davies S, Roberts II LJ (2004) Pyridoxamine: an extremely potent scavenger of 1,4-dicarbonyls. Chem Res Tox 17:410–415
Beeby A, Mohammed DBH, Sodeau JR (1987) Photochemistry and photophysics of glycolaldehyde in solution. J Am Chem Soc 109:857–861
Besler BH, Mertz KM, Kollman PA (1990) Atomic charges derived from semiempirical methods. J Comput Chem 11:431–439
Bohlender JM, Franke S, Stein G, Wolf G (2005) Advanced glycation end products and the kidney. Am J Physiol Renal Physiol 289:645–659
Borch RF, Bernstein MD, Durst HD (1971) The cyanohydridoborate anion as a selective reducing agent. J Am Chem Soc 93:2897–2904
Braun KP, Cody RB, Jones DR, Peterson CM (1995) A structural assignment for a stable acetaldehyde-lysine adduct. J Biol Chem 270:11263–11266
Bunn HF, Higgins PJ (1981) Reaction of monosaccharides with proteins: possible evolutionary significance. Science 213:222–224
Chetyrkin S, Zhang W, Hudson BG, Serianni AS, Voziyan PA (2008a) Pyridoxamine protects proteins from functional damage by 3-deoxyglucosone: mechanism of action of pyridoxamine. Biochemistry 47:997–1006
Chetyrkin S, Mathis ME, Ham A-JL, Hachey DL, Hudson BG, Voziyan PA (2008b) Propagation of protein glycation damage involves modification of tryptophan residues via reactive oxygen species: inhibition by pyridoxamine. Free Radic Biol Med 44:1276–1285
Creighton DJ, Migliorini M, Pourmotabbed T, Guha MK (1988) Optimization of efficiency in glyoxalase pathway. Biochemistry 27:7376–7384
Davies SS, Brantley EJ, Voziyan PA, Amarnath V, Zagol-Ikapitte I, Boutaud O, Hudson BG, Oates JA, Roberts II LJ (2006) Pyridoxamine analogues scavenge lipid-ferived γ-ketoaldehydes and protect against H2O2-mediated cytotoxicity. Biochemistry 45:15756–15767
Dworschák E, Örsi F (1977) Study into the Maillard reaction occurring between methionine and tryptophan on the one hand and glucose on the other. Acta Aliment Acad Sci Hung 6:59–71
Dworkin JP, Miller SL (2000) A kinetic estimate of the free aldehyde content of aldoses. Carbohyd Res 329:359–365
Dyer DG, Blackledge JA, Thrope SR, Baynes JW (1991) Formation of pentosidine during nonenzymatic browning of proteins by glucose. J Biol Chem 266:11654–11660
Glushonok GK, Glushonok TG, Maslovskaya LA, Shadyro OI (2003) A 1H and 13C NMR and UV study of the state of hydroxyacetone in aqueous solutions. Russ J Gen Chem 73:1027–1031
Greenzaid P, Luz Z, Samuel D (1967) A nuclear magnetic resonance study of the reversible hydration of aliphatic aldehydes and ketones. I. Oxygen-17 and proton spectra and equilibrium constants. J Am Chem Soc 89:749–756
Horvat S, Jakas A (2004) Peptide and amino acid glycation: new insights into the Maillard Reaction. J Peptide Sci 10:119–137
Kang Z, Li H, Li G, Yin D (2006) Reaction of pyridoxamine with malondialdehyde: mechanism of inhibition of formation of advanced lipoxidation end-products. Amino acids 30:55–61
Klamt A (1995) Conductor-like screening model for real solvents: a new approach to the quantitative calculation of solvation phenomena. J Phys Chem 99:2224–2235
Kuksis A, Ravandi A, Schneider M (2005) Covalent binding of acetone to aminophospholipids in vitro and in vivo. Ann NY Acd Sci 1043:417–439
Kume S, Takeya M, Mori T, Araki N, Suzuki H, Horiuchi S, Kodama T, Miyauchi Y, Takahashi K (1995) Immunohistochemical and ultrastructural detection of advanced glycation end products in atherosclerotic lesions of human aorta with a novel specific monoclonal antibody. Am J Pathol 147:654–667
Kuzmic P (1996) Program DYNAFIT for the analysis of enzyme kinetic data: application to HIV proteinase. Anal Biochem 237:260–273
Lo TWC, Westwood ME, McLellan AC, Selwood T, Thornalley PJ (1994) Binding and modification of proteins by methylglyoxal under physiological conditions. J Biol Chem 269:32299–32305
Metzler DE, Harris CM, Johnson RJ, Siano DB, Thomson JA (1973) Spectra of 3-hydroxypyridines. Band-shape analysis and evaluation of tautomeric equilibria. Biochemistry 12:5377–5392
Morgan PE, Dean RT, Davies MJ (2002) Inactivation of cellular enzymes by carbonyls and protein-bound glycation/glycoxidation products. Arch Biochem Biophys 403:259–269
Mullarkey CJ, Edelstein D, Brownlee M (1990) Free radical generation by early glycation products: A mechanism for accelerated atherogenesis in diabetes. Biochem Biophys Res Commun 173:932–939
Mulliken RS (1955) Electronic population analysis on LCAO-MO molecular wave functions. I. J Chem Phys 23:1833–1840
Nagai R, Matsumoto K, Ling X, Suzuki H, Araki T, Horiuchi S (2000) Glycolaldehyde, a reactive intermediate for advanced glycation end products, plays an important role in the generation of an active ligand for the macrophage scavenger receptor. Diabetes 49:1714–1723
Nagaraj RH, Sarkar P, Mally A, Biemel KM, Lederer MO, Padayatti PS (2002) Effect of pyridoxamine on chemical modification of proteins bycarbonyls in diabetic rats: characterization of a major product from the reaction of pyridoxamine and methylglyoxal. Arch Biochem Biophys 403:259–269
Nyhammar T, Pernemalm P (1985) Reaction of Nα-Acetyl-DL-tryptophan amide with d-xylose or d-glucose in acidic solution. Food Chem 17:289–296
Onorato JM, Jenkins AJ, Thorpe SR, Baynes JW (2000) Pyridoxamine, an inhibitor of advanced glycation reactions, also inhibits advanced lipoxidation reactions. J Biol Chem 275:21177–21184
Price DL, Rhett PM, Thorpe SR, Baynes JW (2001) Chelating activity of advanced glycation end-product inhibitors. J Biol Chem 276:48967–48972
Reddy VP, Obrenovich ME, Atwood CS, Perry G, Smith MA (2002) Involvement of Maillard reactions in Alzheimer disease. Neurotox Res 4:191–209
Reihl O, Lederer MO, Schwack W (2004) Characterization and detection of lysine-arginine cross-links derived from dehydroascorbic acid. Carbohyd Res 339:483–491
Saito G, Okitani A, Hayase F, Kato H (1986) Characterization of tryptophan derivatives from the reaction of Nα-acetyl-tryptophan with carbonyl compounds. Agric Biol Chem 50:2315–2323
Schuchmann MN, Von Sonntag C (1988) The rapid hydration of the acetyl radical. A pulse radiolysis study of acetaldehyde in aqueous solution. J Am Chem Soc 110:5698–5701
Schweitzer F, Magi L, Mirabel P, George C (1998) Uptake rate measurements of methanesulfonic acid and glyoxal by aqueous droplets. J Phys Chem A 102:593–600
Stadtman ER, Levine RL (2003) Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25:207–218
Stewart JJP (1989) Optimization of parameters for semiempirical methods I. Method. J Comput Chem 10:209–220
Stitt AW (2005) The Maillard reaction in eye diseases. Ann NY Acd Sci 1043:582–597
Thornalley PJ, Langborg A, Minhas HS (1999) Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J 344:109–116
Thorpe SR, Baynes JW (2003) Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids 25:275–281
Ulrich P, Cerami A (2001) Protein glycation, diabetes, and aging. Recent Prog Horm Res 56:1–22
Voziyan PA, Hudson BG (2005) Pyridoxamine as a multifunctional pharmaceutical: targeting pathogenic glycation and oxidative damage. Cell Mol Life Sci 62:1671–1681
Voziyan PA, Metz TO, Baynes JW, Hudson BG (2002) A post-amadori inhibitor pyridoxamine also inhibits chemical modification of proteins by scavenging carbonyl intermediates of carbohydrate and lipid degradation. J Biol Chem 277:3397–3403
Wolff SP, Dean RT (1987) Glucose autoxidation and protein modification. Biochem J 245:243–250
Wolff SP (1993) Diabetes mellitus and free radicals. Br Med Bull 49:642–652
Zeng J, Davies MJ (2005) Evidence for the formation of adducts and S-(carboxymethyl)cysteine on reaction of α-dicarbonyl compounds with thiol groups on amino acids, peptides, and proteins. Chem Res Toxicol 18:1232–1241
Acknowledgments
This work was made possible by a grant from the Spanish Government (DGICYT CTQ 2005-00250) and from the Balearic Government (PROGECIB-28A).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adrover, M., Vilanova, B., Frau, J. et al. A comparative study of the chemical reactivity of pyridoxamine, Ac-Phe-Lys and Ac-Cys with various glycating carbonyl compounds. Amino Acids 36, 437–448 (2009). https://doi.org/10.1007/s00726-008-0098-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-008-0098-6