Abstract
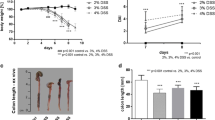
The action of tryptanthrin, a known natural alkaloid, a potent inhibitor of cyclooxygenase COX-2 and 5-lipoxygenase, was studied in a model of dextran sodium sulfate (DSS)-induced mouse colitis by magnetic resonance imaging (MRI). It was shown that the intra-ventricular treatment with tryptanthrin suspensions or emulsion at a dose of 25 mg/kg effectively diminishes lethality at experimental groups and normalizes morphometric parameters of intestine in the treated animals. MR imaging confirmed anti-inflammatory effect of this alkaloid and its capability to decrease the size of bowel walls in animals with colitis.



Similar content being viewed by others
References
E. Von Sommaruga, Justis Libiegs Ann. der Chemie. 195, 302–313 (1879)
P. Friedlander, N. Roschdestwensky, Chem. Ber. 48, 1841–1847 (1915)
F. Schindler, H. Zahner, Arch. Mikrobiol. 79, 187–203 (1971)
A. Sen, S. Mahato, N. Dutta, Tetrahedron Lett. 15, 609–610 (1974)
J. Bergman, B. Egestad, J.O. Lindstrom, Tetrahedron Lett. 18, 2625–2626 (1977)
T. Hosoe, K. Nosawa, N. Kawahara, K. Fukushima, K. Nishimura, M. Miyaji, K. Kawai, Mycopathologia 146, 9–12 (1999)
I. Wagner-Dobler, H. Rheims, A. Felske, A. El-Ghezal, D. Flade-Schroder, H. Laatsch, S. Lang, R. Pukall, B.J. Tindall, Int. J. Syst. Evol. Microbiol. 54, 1177–1179 (2004)
G. Honda, V. Tosirisuk, M. Tabata, Planta Med. 38, 275–276 (1980)
H. Danz, D. Baumann, M. Hamburger, Planta Med. 68, 152–157 (2002)
H. Danz, S. Stoyanova, P. Wippich, A. Brattstrom, M. Hamburger, Planta Med. 67, 411–416 (2001)
V. George, A.S. Koshy, O.V. Singh, M.N.S. Nayar, P. Pushpangadan, Fitoterapia 67, 553–554 (1996)
A.V. Muruganandam, S.K. Bhattacharya, Ind. J. Chem.-Sect. B 39B, 125-131 (2000)
G. Honda, M. Tabata, Planta Med. 36, 85–86 (1979)
M. Yoshikawa, T. Muracami, A. Kishi, T. Sakurama, H. Matsuda, M. Nomura, H. Matsuda, M. Kubo, Chem. Pharm. Bull. 46, 886–888 (1998)
T. Murakami, A. Kishi, T. Sacurama, H. Matsuda, M. Yoshicawa, Heterocycles 54, 957–966 (2001)
J. Bergman, J. Lindstroem, U. Tilstam, Tetrahedron 41, 2879–2881 (1985)
Y. Jahng, Arch. Pharm. Res. 36, 517–535 (2013)
A. Tucker, P. Grundt, Arkivoc. (i) 546-569 (2012)
T. Moskovkina, A. Kalinovskii, V. Makhan’kov, Russ. J. Org. Chem. 48, 123–126 (2012)
T. Moskovkina, M. Denisenko, A. Kalinovskii, V. Stonik, Russ. J. Org. Chem. 49, 1740–1743 (2013)
G. Honda, M. Tabeta, M. Tsuda, Planta Med. 37, 172–174 (1979)
P. Bandekar, K. Roopnarine, V. Parekh, T. Mitchell, M. Novak, R. Sinden, J. Med. Chem. 53, 3558–3565 (2010)
W. Baker, L. Mitscher, U.S. Patent 5, 441, 955 (1995)
L. Mitscher, W. Baker, Med. Res. Rev. 18, 363–374 (1998)
M. Kataoka, K. Hirata, T. Kunikata, S. Ushio, K. Iwaki, K. Ohashi, M. Ikeda, M. Kurimoto, J. Gastroenterol. 36, 5–9 (2001)
A. Bhattacharjee, D. Skanchy, B. Jennings, T. Hudson, J. Brendle, K. Werbovetz, Bioorg. Med. Chem. 10, 1979–1989 (2004)
K. Pitzer, J. Scovill, D. Kyle, L. Gerena. US Patent, 6,531,487 (2003)
J. Scovill, E. Blank, M. Konnick, E. Nenortas, T. Shapiro, Antimicr. Agents Chemotherap. 46, 882–883 (2002)
H. Chan, H. Yip, N. Mak, K. Leung, Cell. Molecul. Immunol. 6, 335–342 (2009)
C. Oberthuer, R. Jaeggi, M. Hamburger, Fitoterapia 76, 324–332 (2005)
M. Micallef, K. Iwaki, T. Ishihara, S. Ushio, M. Aga, T. Kunikata, S. Koya-Miayata, T. Kimoto, M. Ikeda, M. Kurimoto, Int. Immunopharmacol. 2, 565–578 (2002)
T. Ishihara, K. Kohno, S. Ushio, K. Iwaki, M. Ikeda, M. Kurimoto, Eur. J. Pharmacol. 407, 197–204 (2000)
J. Terrah, P. Olson, R. Halberg, Experimental Small Animal Colonoscopy. ed. P. Misouitz, Medicine/Gasteroenterology/Colonoscopy. 297 p. (2007) doi:10.5772/21573
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agafonova, I.G., Moskovkina, T.V. Studies on Anti-Inflammatory Action of Tryptanthrin, Using a Model of DSS-Induced Colitis of Mice and Magnetic Resonance Imaging. Appl Magn Reson 46, 781–791 (2015). https://doi.org/10.1007/s00723-015-0674-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-015-0674-3