Abstract
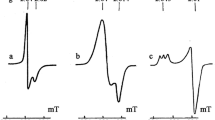
Some recent data on the presence of mononuclear dinitrosyl iron complexes (M-DNIC) with persulfide (R-S-S−) ligands with a characteristic electron paramagnetic resonance signal at g ⊥ = 2.35 and g || = 2.02 (g aver. = 2.03) in biological systems (e.g., Escherichia coli cells and isolated iron–sulfur proteins) are reviewed. The generation of M-DNIC is controlled by inorganic sulfur (sulfide, S2−) whose binding to thiols gives persulfides. It is suggested that enhanced production of inorganic sulfur is a result of destruction of active centers of iron–sulfur proteins in the presence of NO or NO-containing compounds. Dinitrosyl iron complexes with thiol-containing ligands are the most active participants in this process. Inorganic sulfur may appear in biological systems during the synthesis or resynthesis of active centers of iron–sulfur proteins in response to activation of cysteine desulfurase, the key enzyme in sulfide synthesis from cysteine.







Similar content being viewed by others
References
R.M. Nalbandyan, A.F. Vanin, L.A. Blumenfeld, in Abstracts of the Meeting “Free Radicals Processes in Biological Systems”, Moscow, (1964) p. 18
J.R. Mallard, M. Kent, Nature 204, 1192 (1964)
A.F. Vanin, R.M. Nalbandyan, Biofizika (Rus) 10, 167–168 (1965)
A.J. Vithaythil, J.L. Ternberg, B. Commoner, Nature 207, 1246–1249 (1965)
A.F. Vanin, L.A. Blumenfeld, A.G. Chenverokov, Biofizika (Rus) 12, 829–841 (1967)
E.N. Frolov, A.F. Vanin, Biofizika (Rus) 18, 605–610 (1973)
W.A. Goodman, I.B. Raynor, M.C.R. Simmons, J. Chem. Soc. (A). 2038–2043 (1970)
H. Cruz-Ramos, J. Crack, G. Wu, M.N. Hughes, C. Scott, A.J. Thomson, J. Green, R.K. Poole, EMBO J. 21, 3235–3244 (2002)
J. Crack, L.J. Smith, M.R. Stapleton, J. Peck, N.J. Watmough, M.J. Buttner, R.S. Buxton, V.S. Oganesyan, A.J. Thomson, N.E. Le Brun, J. Am. Chem. Soc. 133, 1112–1121 (2011)
J. Crack, M.R. Stapleton, J. Green, A.J. Thomson, N.E. Le Brun, J. Biol. Chem. 288, 11492–11502 (2013)
I.I. Lobysheva, M.V. Stupakova, V.D. Mikoyan, S.V. Vasilyeva, A.F. Vanin, FEBS Lett. 454, 177–180 (1999)
C.F. Amabile-Cuevas, B. Demple, Nucleic Acids Res. 99, 4479–4484 (1991)
N.V. Voevodskaya, V.A. Serezhenkov, C.E. Cooper, L.N. Kubrina, A.F. Vanin, Biochem. J. 368, 633–639 (2002)
S.V. Vasilyeva, EYu. Moshkovskaya, A.S. Terekhov, V.D. Mikoyan, A.F. Vanin, Russ. J. Genetics 44, 21–26 (2008). (Translation from Genetika)
S.V. Vasilyeva, D.A. Strel`tsova, A.V. Vlaskina, V.D. Mikoyan, A.F. Vanin, Biophysics 57, 166–169 (2012). (Translation from Biofizika)
S.V. Vasilyeva, D.A. Strel`tsova, E.Yu. Moshkovskaya, A.F. Vanin, V.D. Mikoyan, N.A. Sanina, S.M. Aldoshin, Dokl. Biochem. Biophys. 435, 283–286 (2010). (Translation from Doklady Akademii Nauk)
M.V. Stupakova, I.I. Lobysheva, V.D. Mikoyan, A.F. Vanin, S.V. Vasilyeva, Biokhimiya 65(65), 810–816 (2000)
H. Ding, B. Demple, Proc. Natl. Acad. Sci. USA 97, 5146–5150 (2000)
S.V. Vasilyeva, M.V. Stupakova, I.I. Lobysheva, V.D. Mikoyan, A.F. Vanin, Biochemistry (Moscow) 66, 984–988 (2001). (Translation from Biokhimiya)
S.V. Vasilyeva, M.V. Stupakova, I.I. Lobysheva, V.D. Mikoyan, A.F. Vanin, Biokhimiya (Rus) 66, 1209–1214 (2001)
A.F. Vanin, I.V. Malenkova, V.A. Serezhenkov, Nitric Oxide Biol. Chem. 1, 191–203 (1997)
S. Constanzo, S. Menage, R. Purello, R.P. Bonomo, M. Fontecave, Inorg. Chim. Acta. 318, 1–7 (2001)
A.F. Vanin, N.A. Sanina, V.A. Serezhenkov, DSh Burbaev, V.I. Lozinsky, S.M. Aldoshin, Nitric Oxide Biol. Chem. 16, 82–93 (2007)
A.F. Vanin, A.P. Poltorakov, V.D. Mikoyan, L.N. Kubrina, DSh Burbaev, Nitric Oxide Biol. Chem. 23, 1236–1249 (2010)
A.F. Vanin, D.Sh. Burbaev, J. Biophys. 2011, Article ID 878236 (2011)
J.S. Hickok, S. Sahni, H. Shen, A. Arvind, C. Antoniou, L.W.M. Fung, D.D. Thomas, Free Rad. Biol. Med. 51, 1558–1566 (2011)
C.E. Tinberg, Z.J. Tonzetich, H. Wang, L.H. Do, Y. Yoda, S.P. Cramer, S.J. Lippard, J. Am. Chem. Soc. 132, 18168–18176 (2010)
N.P. Tucker, M.G. Hicks, T.A. Clarke, J.C. Crack, G. Chandra, N.E. Le Brun, R. Dixon, M.I. Hutchison, PLoS One 3, e3623 (2008)
R.R. Borodulin, L.N. Kubrina, V.A. Serezhenkov, D.S. Burbaev, V.D. Mikoyan, A. Vanin, Nitric Oxide Biol. Chem. 35, 35–41 (2013)
A.F. Vanin, Nitric Oxide Biol. Chem. 21, 1–13 (2009)
A.F. Vanin, Open Conf. Proc. J. 4, 31–37 (2013)
H. Kimura, N. Shibuya, Y. Kimura, Antioxid. Redox Signal. 17, 45–57 (2012)
Q. Li, J.R. Lancaster, Nitric Oxide Biol. Chem. 35, 21–34 (2013)
Acknowledgments
This work has been supported by the Russian Foundation for Basic Research (project no. 12-04-00346a) and by the Presidium of the Russian Academy of Sciences within the framework “Fundamental Sciences for Medicine (2013)”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vanin, A.F., Vasilyeva, S.V., Streltsova, D.A. et al. EPR Characterization of Mononuclear Dinitrosyl Iron Complex with Persulfide as a New Representative of Dinitrosyl Iron Complexes in Biological Systems: an Overview. Appl Magn Reson 45, 375–387 (2014). https://doi.org/10.1007/s00723-014-0523-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-014-0523-9