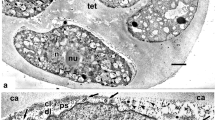
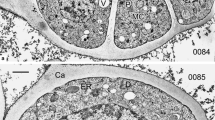
Abstract
Our aim was to unravel the underlying mechanisms of pollen wall development in Cymbalaria muralis. By determining the sequence of developing substructures with TEM, we intended to compare it with that of other taxa and clarify whether physical processes of self-assembly and phase separation were involved. In parallel, we tried to simulate in vitro the substructures observed in Cymbalaria muralis exine development, using colloidal mixtures, to determine whether purely physical self-assembly processes could replicate them. Exine ontogeny followed the main stages observed in many other species and was initiated by phase separation, resulting in heterogeneity of the homogeneous contents of the periplasmic space around the microspore which is filled with genome-determined substances. At every stage, phase separation and self-assembly come into force, gradually driving the substances through the sequence of mesophases: spherical micelles, columns of spherical micelles, cylindrical micelles arranged in a layer, laminate micelles. The final two of these mesophases define the structure of the columellate ectexine and lamellate endexine respectively. Structures obtained in vitro from colloidal mixtures simulated the developing exine structures. Striking columella-like surface of some abnormal tapetal cells and lamella-like structures in the anther medium confirm the conclusion that pattern generation is a feature of colloidal materials, after genomic control on material contents. Simulation experiments show the high pattern-generating capacity of colloidal interactions.










Similar content being viewed by others
Data availability
All relevant data are included in this manuscript and its supporting information is acceptable if this is the case.
References
Alberti S (2017) Phase separation in biology. Curr Biol 27:R1089–R1107. https://doi.org/10.1016/j.cub.2017.08.069
Ariizumi T, Toriyama K (2011) Genetic regulation on sporopollenin synthesis and pollen exine development. Annu Rev Plant Biol 62:1–24. https://doi.org/10.1146/annurev-arplant-042809-112312
Ariizumi T, Hatakeyama K, Hinata K, Inatsugi R, Nishida I, Sato S, Kato T, Tabata S, Toriyama K (2004) Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J 39:170–181. https://doi.org/10.1111/j.1365-313X.2004.02118.x
Bayer O, Hoffman H, Ulbricht W, Thurn H (1986) The influence of solubilized additives on surfactant solutions with rodlike micelles. Adv Colloid Interface Sci 26:177–203. https://doi.org/10.1016/0001-8686(86)80021-5
Benítez M (2013) An interdisciplinary view on dynamic models for plant genetics and morphogenesis: scope, examples and emerging research avenues. Front Plant Sci 4:7. https://doi.org/10.3389/fpls.2013.00007
Biggs S, Kline SR, Walker LM (2004) The adsorption of polymerized rod-like micelles at the solid-liquid interface. Langmuir 20:1085–1094
Blackmore S, Skvarla JJ (2012) John Rowley (1926–2010), palynologist extraordinaire. Grana 51(2):77–83. https://doi.org/10.1080/00173134.2012.661454
Blackmore S, Wortley AH, Skvarla JJ, Rowley JR (2007) Pollen wall development in flowering plants. New Phytol 174:483–498. https://doi.org/10.1111/j.1469-8137.2007.02060
Blackmore S, Wortley AH, Skvarla JJ, Gabarayeva NI, Rowley JR (2010) Developmental origins of structural diversity in pollen walls of Compositae. Plant Syst Evol 284:17–32. https://doi.org/10.1007/s00606-009-0232-2
Blankschtein D, Thurston GM, Benedek GB (1985) Theory of phase separation in micellar solutions. Phys Rev Lett 54:955–958
Collinson ME, Hemsley AR, Taylor WA (1993) Sporopollenin exhibiting colloidal organization in spore walls. Grana Suppl 1:31–39
Dickinson HG, Sheldon JM (1986) The generation of patterning at the plasma membrane of the young microspore of Lilium. In: Blackmore S, Ferguson IK (eds) Pollen and spores: form and function. Academic Press, London, pp 1–18
Dobritsa AA, Geanconteri A, Shrestha J et al (2011) A large-scale genetic screen in Arabidopsis to identify genes involved in pollen exine production. Plant Physiol 157:947–970. https://doi.org/10.1104/pp.111.179523
Gabarayeva N, Grigorjeva V (2016) Simulation of exine patterns by self-assembly. Plant Syst Evol 302:1135–1156. https://doi.org/10.1007/s00606-016-1322-6
Gabarayeva NI, Grigorjeva VV (2021) An integral insight into pollen wall development: involvement of physical processes in exine ontogeny in Calycanthus floridus L., with an experimental approach. Plant J 105:736–753. https://doi.org/10.1111/tpj.15070
Gabarayeva NI, Hemsley AR (2006) Merging concepts: the role of self-assembly in the development of pollen wall structure. Rev Palaeobot Palynol 138:121–139. https://doi.org/10.1016/j.revpalbo.2005.12.001
Gabarayeva NI, Grigorjeva VV, Rowley JR (2010) A new look at sporoderm ontogeny in Persea americana. Micelles and the hidden side of development. Ann Bot 105:939–955. https://doi.org/10.1093/aob/mcq075
Gabarayeva N, Grigorjeva V, Polevova S (2014) Sporoderm and tapetum ontogeny in Juniperus communis (Cupressaceae). Connective structures between tapetum and microspores. Rev Palaeobot Palynol 206:23–44. https://doi.org/10.1016/j.revpalbo.2014.03.004
Gabarayeva N, Grigorjeva V, Blackmore S (2016) Pollen wall substructure and development in Tanacetum vulgare (Compositae: Anthemideae): revisiting hypotheses on pattern formation in complex cell walls. Int J Plant Sci 177(4):347–370. https://doi.org/10.1086/684946
Gabarayeva N, Grigorjeva V, Polevova S, Hemsley AR (2017) Pollen wall and tapetum development in Plantago major (Plantaginaceae): assisting self-assembly. Grana 56(2):1–31. https://doi.org/10.1080/00173134.2016.1159729
Gabarayeva NI, Polevova SV, Grigorjeva VV, Blackmore S (2018) Assembling the thickest plant cell wall: exine development in Echinops (Asteraceae, Cynareae). Planta 248(2):323–346. https://doi.org/10.1007/s00425-018-2902-1
Gabarayeva NI, Grigorjeva VV, Shavarda AL (2019) Mimicking pollen and spore walls: self-assembly in action. Ann Bot 123:1205–1218. https://doi.org/10.1093/aob/mcz027
Gabarayeva NI, Grigorjeva VV, Lavrentovich MO (2020) Artificial pollen walls simulated by the tandem processes of phase separation and self-assembly in vitro. New Phytol 225:1956–1973. https://doi.org/10.1111/nph.16318
Gabarayeva NI, Polevova SV, Grigorjeva VV, Hiscock SJ (2021) Underlying mechanisms of development: pollen wall ontogeny in Chloranthus japonicus and a reconsideration of pollen ontogeny in early-diverging lineages of angiosperms. Bot J Linn 196(2):221–224. https://doi.org/10.1093/botlinnean/boaa102
Gerasimova-Navashina EN (1973) Physico-chemical nature of primexine formation of angiosperm pollen grains. In: Kovarski A (ed) Embryology of angiosperms. Ştiinţǎ, Kishinev, p 57–70
Grigorjeva VV, Polevova SV, Gabarayeva NI (2021) Pollen wall development in Hydrangea bretschneiderii Dippel. (Hydrangeaceae): advanced interpretation through physical input, with in vitro experimental verification. Protoplasma 258:431–447. https://doi.org/10.1007/s00709-020-01571-4
Griffiths PC, Hemsley AR (2002) Raspberries and muffins – mimiking biological pattern formation. Colloids Surf B Biointerfaces 25:163–170. https://doi.org/10.1016/S0927-7765(01)00316-2
Gubatz S, Wiermann R (1992) Studies on sporopollenin biosynthesis in Tulipa anthers. 3. Incorporation of specifically labeled C-14 Phenylalanine in comparison to other precursors. Bot Acta 105:407–413. https://doi.org/10.1111/j.1438-8677.1992.tb00321.x
Hemsley AR (1998) Nonlinear variation in simulated complex pattern development. J Theor Biol 192(1):73–79. https://doi.org/10.1006/jtbi.1997.0610
Hemsley AR, Collinson ME, Brain APR (1992) Colloidal crystal-like structure of sporopollenin in the megaspore walls of recent Selaginella and similar fossil spores. Bot J Linn Soc 108:307–320. https://doi.org/10.1111/j.1095-8339.1992.tb00247.x
Hemsley AR, Gabarayeva NI (2007) Exine development: the importance of looking through a colloid chemistry “window.” Plant Syst Evol 263:25–49. https://doi.org/10.1007/s00606-006-0465-2
Hemsley AR, Griffiths PC (2000) Architecture in the microcosm: biocolloids, self-assembly and pattern formation. Phil Trans Royal Soc London A 358:547–564
Hemsley AR, Jenkins PD, Collinson ME, Vincent B (1996) Experimental modelling of exine self-assembly. Bot J Linn Soc 121:177–187. https://doi.org/10.1006/bojl.1996.0031
Hemsley AR, Vincent B, Collinson ME, Griffiths PC (1998) Simulated self-assembly of spore exines. Ann Bot 82:105–109. https://doi.org/10.1006/anbo.1998.0653
Hemsley AR, Griffiths PC, Mathias R, Moore SEM (2003) A model for the role of surfactants in the assembly of exine sculpture. Grana 42:38–42. https://doi.org/10.1080/00173130310008562
Hemsley AR, Lewis J, Griffiths PC (2004) Soft and sticky development: some underlying reasons for microarchitectural pattern convergence. Rev Palaeobot Palynol 130:105–119. https://doi.org/10.1016/j.revpalbo.2003.12.004
Heslop-Harrison J (1971) The pollen wall: structure and development. In: Heslop-Harrison J (ed) Pollen development and physiology. Butterworths, London, pp 75–98. https://doi.org/10.1016/B978-0-408-70149-5.50013-0
Heslop-Harrison J (1972) Pattern in plant cell walls: morphogenesis in miniature. Proc R Inst G B 45:335–351
Honda S, Koga M, Tokita M, Yamamoto T, Tezuka Y (2015) Phase separation and self-assembly of cyclic amphiphilic block copolymers with a main-chain liquid crystalline segment. Polym Chem 6:4167–4176. https://doi.org/10.1039/C5PY00346F
Hyde ST, Schröder GE (2003) Novel surfactant mesostructural topologies: between lamellae and columnar (hexagonal) forms. Curr Opin Colloid Interface Sci 8:5–14. https://doi.org/10.1016/S1359-0294(03)00014-1
Hyman AA, Weber CA, Jülicher F (2014) Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol 30:39–58. https://doi.org/10.1146/annurev-cellbio-100913-013325
Ingber D (1993) Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci 104:613–627
Kauffman SA (1993) The origin of order. Oxford University Press, New York
Kurakin A (2005) Self-organization versus watchmaker: stochastic dynamics of cellular organization. Biol Chem 386:247–254. https://doi.org/10.1515/BC.2005.030
Lavrentovich MO, Horsley EM, Radja A, Sweeney AM, Kamien RD (2016) First-order patterning transitions on a sphere as a route to cell morphology. PNAS USA 113:5189–5194. https://doi.org/10.1073/pnas.1600296113
Lecuit T (2008) “Developmental mechanics”: cellular patterns controlled by adhesion, cortical tension and cell division. HFSP J 2:72–78. https://doi.org/10.2976/1.2896332
Lecuit T, Lenne PF (2007) Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol 8:633–644. https://doi.org/10.1038/nrm2222
Lintilhac PM (2014) The problem of morphogenesis: unscripted biophysical control systems in plants. Protoplasma 251:25–36. https://doi.org/10.1007/s00709-013-0522-y
Li J, Yu M, Geng LL, Zhao J (2010) The fasciclin-like arabinogalactan protein gene, FLA3, is involved in microspore development of Arabidopsis. Plant J 64:482–497. https://doi.org/10.1111/j.1365-313X.2010.04344.x
Li W, Liu Y, Douglas C (2017) Role of glycosyltransferases in pollen wall primexine formation and exine patterning. Plant Physiol 173:167–182. https://doi.org/10.1104/pp.16.00471
Li F-S, Phyo P, Jacobowitz J, Hong M, Weng J-K (2019) The molecular structure of plant sporopollenin. Nat Plants 5:41–46. https://doi.org/10.1038/s41477-018-0330-7
Liu Z, Lin S, Shi J, Yu J, Zhu L, Yang X, Zhang D, Liang W (2017) Rice No Pollen 1 (NP1) is required for anther cuticle formation and pollen exine patterning. Plant J 91(2):263–277. https://doi.org/10.1111/tpj.13561
MacDougall AJ, Rigby NM, Ring SG (1997) Phase separation of plant cell wall polysaccharides and its implication for cell wall assembly. Plant Physiol 114:353–362. https://doi.org/10.1104/pp.114.1.353
Mandelbrot BB (1982) The fractal geometry of nature. WH Freeman and Co, San Francisco
Minelli A (2019) An evo-devo perspective on analogy in biology. Philosophies 4(1):5. https://doi.org/10.3390/philosophies4010005
Mitrea DM, Kriwacki RW (2016) Phase separation in biology; functional organization of a higher order. J Cell Commun Signal 14:1. https://doi.org/10.1186/s12964-015-0125-7
Mondol P, Xu D, Duan L, Shi J, Wang C, Chen X, Chen M, Hu J, Liang W, Zhang D (2020) Defective pollen wall 3 (DPW3), a novel alpha integrin-like protein, is required for pollen wall formation in rice. New Phytol 225:807–822. https://doi.org/10.1111/nph.16161
Moore SEM, Gabarayeva N, Hemsley AR (2009) Morphological, developmental and ultrastructural comparison of Osmunda regalis L. spores with spore mimics. Rev Paleobot Palynol 156:177–184
Pettitt JM (1979) Ultrastructure and cytochemistry of spore wall morphogenesis. In: Dyer AF (ed) The experimental biology of ferns. Acad Press, London, pp 211–252
Pettitt JM, Jermy AC (1974) The surface coats on spores. Biol J Linn Soci 6:245–257. https://doi.org/10.1111/j.1095-8312.1974.tb00723.x
Quilichini TD, Douglas CJ, Samuels AL (2014) New views of tapetum ultrastructure and pollen exine development in Arabidopsis thaliana. Ann Bot 114:1189–201. https://doi.org/10.1093/aob/mcu042
Quilichini TD, Grienenberger E, Douglas CJ (2015) The biosynthesis, composition and assembly of the outer pollen wall: a tough case to crack. Phytochemistry 113:170–182. https://doi.org/10.1016/j.phytochem.2014.05.002
Radja A (2020) Pollen wall patterns as a model for biological self-assembly. J Exp Zool B Mol Dev Evol 1–13. https://doi.org/10.1002/jez.b.23005
Radja A, Horsley EM, Lavrentovich MO, Sweeney AM (2019) Pollen patterns form from modulated phases. Cell 176:856–868. https://doi.org/10.1016/j.cell.2019.01.014
Routray P, Li T, Yamasaki A, Yoshinari A, Takano J, Choi W, Sams C, Robertsa D (2018) Nodulin intrinsic protein 7;1 is a tapetal boric acid channel involved in pollen cell wall formation. Plant Physiol 178:1269–1283. https://doi.org/10.1104/pp.18.00604
Rowley JR (1973) Formation of pollen exine bacules and microchannels on a glycocalyx. Grana 13:129–138. https://doi.org/10.1080/00173137309429889
Rowley JR (1990) The fundamental structure of the pollen exine. Plant Syst Evol Suppl 5:13–29. https://doi.org/10.1007/978-3-7091-9079-1_2
Rowley JR, Dahl AO (1977) Pollen development in Artemisia vulgaris with special reference to glycocalyx material. Pollen Spores 19:169–284
Rowley JR, Dunbar A (1970) Transfer of colloid iron from sporophyte to gametophyte. Pollen Spores 12:305–328
Rowley JR, Skvarla JJ, Walles B (1999) Microsporogenesis in Pinus sylvestris. VII. Exine expantion and tapetal development. Taiwania 44:325–344. https://doi.org/10.6165/tai.1999.44(3).325
Scott RJ (1994) Pollen exine – the sporopollenin enigma and the physics of pattern. In: Scott RJ, Stead MA (eds) Molecular and cellular aspects of plant reproduction. Society for Experimental Biology Seminar Series 55. Cambridge Univ. Press, p 49–81
Sheldon JM, Dickinson HG (1983) Determination of patterning in the pollen wall of Lilium henryi. J Cell Sci 63:191–208
Suzuki T, Narciso J, Zeng W, van de Meene A, Yasutomi M, Takemura S, Lampugnani E, Doblin M, Bacic A, Ishiguro S (2017) KNS4/UPEX1: a type II arabinogalactan β-(1,3)-galactosyltransferase required for pollen exine development. Plant Physiol 173:183–205. https://doi.org/10.1104/pp.16.01385
Taylor ML, Osborn JM (2006) Pollen ontogeny in Brasenia (Cabombaceae, Nymphaeales). Am J Bot 93:344–356. https://doi.org/10.3732/ajb.93.3.344
Taylor ML, Hudson PJ, Rigg JM, Strandquist JN, Green JS, Thiemann TC, Osborn JM (2013) Pollen ontogeny in Victoria (Nymphaeales). Int J Plant Sci 174:1259–1276. https://doi.org/10.1086/673246
Taylor M, Cooper RL, Schneider EL, Osborn JM (2015) Pollen structure and development in Nymphaeales: insights into character evolution in an ancient angiosperm lineage. American J Bot 102:1–18. https://doi.org/10.3732/ajb.1500249
Thompson DA (1917) On growth and form. Cambridge University Press, Cambridge
Van Uffelen GA (1991) The control of spore wall formation. In: Blackmore S, Barnes SH (eds) Pollen and spores: patterns of diversification. Clarendon Press, Oxford, pp 89–102
Wang R, Dobritsa A (2018) Exine and aperture patterns on the pollen surface: their formation and roles in plant reproduction. Annu Plant Rev 1:1–40. https://doi.org/10.1002/9781119312994.apr0625
Wang R, Dobritsa A (2021) Loss of THIN EXINE2 disrupts multiple processes in the mechanism of pollen exine formation. Plant Physiol 187:133–157. https://doi.org/10.1093/plphys/kiab244
Wang R, Owen HA, Dobritsa A (2021) Dynamic changes in primexine during the tetrad stage of pollen development. Plant Physiol kiab426, 1–12. https://doi.org/10.1093/plphys/kiab426
Weber M (1992) the formation of pollenkitt in Apium nodiflorum (Apiaceae). Ann Bot 70:573–577. https://doi.org/10.1093/oxfordjournals.aob.a088519
Wilmesmeier S, Wiermann R (1997) Immunocytochemical localization of phenolic compounds in pollen walls using antibodies against p-coumaric acid coupled to bovine serum albumin. Protoplasma 197:148–159
Wodehouse RP (1935) Pollen grains: their structure, identification and significance in science and medicine. McGraw-Hill Co., New York
Acknowledgements
This work was carried out in the framework of the institutional research project of the Komarov Botanical Institute of the Russian Academy of Sciences ‘Pollen and spores of living and fossil plants: morphology and development’ no. AAAA-A19-119080790048-7 on the equipment of the Core Facility ‘Cellular and Molecular Technologies in Plant Science’ of the Komarov Botanical Institute (Saint Petersburg). Special thanks go to Stephen Blackmore for advices and help in the course of the preparation of the manuscript. We are grateful to our anonymous reviewers for their thoughtful and detailed reviews.
Funding
Institutional research project of the Komarov Botanical Institute of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Contributions
SVP: collecting and fixation of C. miralis material and preparing of ultrathin sections; VVG: fixations and embedding of models’ samples and preparing ultrathin sections, staining of all sections and taking a part of TEM pictures; NIG: design and performance of the modelling experiments, taking a part of TEM pictures, the principal analysis and writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Benedikt Kost
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Polevova, S.V., Grigorjeva, V.V. & Gabarayeva, N.I. Pollen wall and tapetal development in Cymbalaria muralis: the role of physical processes, evidenced by in vitro modelling. Protoplasma 260, 281–298 (2023). https://doi.org/10.1007/s00709-022-01777-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-022-01777-8