Abstract
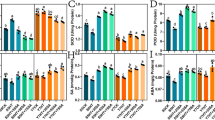
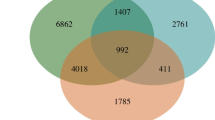
Among stresses, heat stress (HS) is a prime factor restricting plant growth and productivity. However, the molecular mechanisms of plants’ response to HS need to be further uncovered. Here, the transcriptome response of maize seedlings to HS was dissected using transcriptome data analysis. The data exhibited that a total of 43,221 genes in maize seedlings had been found, 37,534 of which were referred, while 5686 were not. Under HS, comparison with the control without HS, there were 13,607 genes that were differentially expressed (DEGs, 6195 upregulated and 7412 downregulated). In addition, Gene Ontology (GO) enrichment analysis indicated that there were 220, 478, and 1300 terms that were enriched in cellular component, molecular function, and biological process, respectively. Significantly enriched GO terms were involved in 23 cellular components, 27 molecular functions, and 124 biological processes. Also, Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis suggested that there were 2613 DEGs that were assigned to 131 pathways, 14 of which (enriched 1068 DEGs in total) were significantly upregulated. These pathways were mainly related to protein renaturation, biomembrane repair, osmotic adjustment, and redox balance. Among them, protein processing in endoplasmic reticulum was the most significantly upregulated. The transcriptome data decoded that protein renaturation, biomembrane repair, osmotic adjustment, and redox balance played a key role in the response of maize seedlings to HS.










Similar content being viewed by others
References
Ali S, Rizwan M, Arif MS, Ahmad R, Hasanuzzaman M, Ali B, Hussain A (2020) Approaches in enhancing thermotolerance in plants: an updated review. J Plant Growth Regul 39:456–480
Ashburner M, Ball CA, Blake JA, Botstein J, Butler H (2000) Gene ontology: tool for the unification of biology. Nat Genetics 25(1): 25
Chen S, Zhou Y, Chen Y, Fu J (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. bioRxiv, 274100
Ding Y, Shi Y, Yang S (2020) Molecular regulation of plant response to environmental temperatures. Mol Plant 13:544–564
Figueroa-Soto CG, Valenzuela-Soto EM (2018) Glycine betaine rather than acting only as an osmolyte also plays a role as regulator in cellular metabolism. Biochimie 147:89–97
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155:93–100
Frey FP, Urbany C, Hüttel B, Reinhardt R, Stich B (2015) Genome-wide expression profiling and phenotypic evaluation of European maize inbreds at seedling stage in response to heat stress. BMC Genomics 16:123
Gonzalez-Schain N, Dreni L, Lawas LMF, Galbiati M, Colombo L, Heuer S, Jagadish KSV, Kater MM (2016) Genome-wide transcriptome analysis during anthesis reveals new insights into the molecular basis of heat stress responses in tolerant and sensitive rice varieties. Plant Cell Physiol 57:57–68
Hasanuzzaman M, Fotopoulos V (2019) Priming and pretreatment of seeds and seedlings. Springer, Singapore
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684
Hasanuzzaman M, Anee TI, Bhuiyan TF, Nahar K, Fujita M (2019) Emerging role of osmolytes in enhancing abiotic stress tolerance in rice. Advances in rice research for abiotic stress tolerance. Elsevier, Kidlington, pp 677–708
Kanehisa M, Goto S (2000) KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28(1):27–30
Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12(4):357
Kollist H, Zandalinas SI, Sengupta S, Nuhkat M, Kangasjärvi J, Mittler R (2019) Rapid responses to abiotic stress: priming the landscape for the signal transduction network. Trends Plant Sci 24:25–37
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie2. Nat Methods 9(4):357–359
Lawas LMF, Zuther E, Jagadish SVK, KHincha D (2018) Molecular mechanisms of combined heat and drought stress resilience in cereals. Curr Opin Plant Biol 45:212–217
Leipner J, Stamp P (2009) Chilling stress in maize seedlings. In: Bennetzen JL, Hake SC (eds) Handbook of Maize: Its Biology. Springer, Berlin, pp 291–344
Li ZG (2021) Role of methylglyoxal and its detoxification systems in plant thermotolerance. Acta Physiol Plant, in press
Li ZG, Yang SZ, Long WB, Yang GX, Shen ZZ (2013a) Hydrogen sulfide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ 36:1564–1572
Li ZG, Ding XJ, Du PF (2013b) Hydrogen sulfide donor sodium hydrosulfide-improved heat tolerance in maize and involvement of proline. J Plant Physiol 170:741–747
Li ZG, Xie LR, Li XJ (2015) Hydrogen sulfide acts as a downstream signal molecule in salicylic acid-induced heat tolerance in maize (Zea mays L.) seedlings. J Plant Physiol 177:121–127
Li ZG, Ye XY, Qiu XM (2019) Glutamate signaling enhances the heat tolerance of maize seedlings by plant glutamate receptor-like channels-mediated calcium signaling. Protoplasma 256:1165–1169
Li ZG, Xiang RH, Wang JQ (2021) Hydrogen sulfide–phytohormone interaction in plants ender physiological and stress conditions. J Plant Growth Regul, in press
Li J, Zhang J, Jia H, Yue Z, Lu M, Xin X, Hu J (2018) Genome-wide characterization of the sHsp gene family in Salix suchowensis reveals its functions under different abiotic stresses. Int J Mol Sci 19:3246
Lobell DB, Schlenker W, Costa-Roberts J (2011) Climate trends and global crop production since 1980. Science 333(6042):616–620
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12): 550
Mangelsen E, Kilian J, Harter K, Jansson C, Wanke D, Sundberg E (2011) Transcriptome analysis of high-temperature stress in developing barley caryopses: early stress responses and effects on storage compound biosynthesis. Mol Plant 4:97–115
Margutti MP, Reyna M, Vilchez CA, Villasuso AL (2019) Lipid profiling shows tissue-specific differences in barley for glycerolipid composition in response to chilling. Environ Exp Bot 158:150–160
Mishra D, Shekhar D, Singh D, Chakraborty S, Chakraborty N (2018) Heat shock proteins and abiotic stress tolerance in plants. In: Asea AAA, Kaur P (eds) Regulation of heat shock protein responses. Springer, Cham, pp 41–69
Nadarajah KK (2020) ROS Homeostasis in abiotic stress tolerance in plants. Int J Mol Sci 21(15):5208
Niu Y, Xiang Y (2018) An overview of biomembrane functions in plant responses to high-temperature stress. Front Plant Sci 9:915
Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K (2017) Transcriptional regulatory network of plant heat stress response. Trends Plant Sci 22:53–65
Pandey S (2019) Heterotrimeric G-protein signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 70:213–238
Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT (2015) StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat biotech 33(3): 290
Pertea M, Kim D, Pertea GM, Leek JT, Salzber SL (2016) Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc 11(9): 1650
Qian Y, Ren Q, Zhang J, Chen L (2019) Transcriptomic analysis of the maize (Zea mays L.) inbred line B73 response to heat stress at the seedling stage. Gene 692:68–78
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1):139–140
Sakr S, Wang M, Dédaldéchamp F, Perez-Garcia MD, Ogé L, Hamama L, Atanassova R (2018) The sugar-signaling hub: overview of regulators and interaction with the hormonal and metabolic network. Int J Mol Sci 19:2506
Savvides A, Ali S, Tester M, Fotopoulos V (2016) Chemical priming of plants against multiple abiotic stresses: mission possible? Trends Plant Sci 21:329–340
Shi J, Yan B, Lou X, Ma H, Ruan S (2017) Comparative transcriptome analysis reveals the transcriptional alterations in heat-resistant and heat-sensitive sweet maize (Zea mays L.) varieties under heat stress. BMC Plant Biol 17:26
Strable J, Scanlon MJ (2009) Maize (Zea mays): a model organism for basic and applied research in plant biology. Cold Spring Harb Protoc; 10(2009)pdb.emo132.
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wang Y, Ye XY, Qiu XM, Li ZG (2019) Methylglyoxal triggers the heat tolerance in maize seedlings by driving AsA-GSH cycle and reactive oxygen species-/methylglyoxal-scavenging system. Plant Physiol Biochem 138:91–99
Xu ZS, Li ZY, Chen Y, Chen M, Li LC, Ma YZ (2012) Heat shock protein 90 in plants: molecular mechanisms and roles in stress responses. Int J Mol Sci 13:15706–15723
Yadav DK, Islam SMS, Tuteja N (2012) Rice heterotrimeric G-protein gamma subunits (RGG1 and RGG2) are differentially regulated under abiotic stress. Plant Signal Behav 7:733–740
Yang L, Wen KS, Ruan X, Zhao YX, Wei F, Wang Q (2018) Response of plant secondary metabolites to environmental factors. Molecules 23:762
Ye XY, Qiu XM, Sun YY, Li ZG (2020) Interplay between hydrogen sulfide and methylglyoxal initiates thermotolerance in maize seedlings by modulating reactive oxidative species and osmolyte metabolism. Protoplasma 257:1415–1432
Yoon J, Cho LH, Tun W, Jeon JS, An G (2021) Sucrose signaling in higher plants. Plant Sci 302:110703
Zhao J, Lu Z, Wang L, Jin B (2021) Plant responses to heat stress: physiology, transcription, noncoding RNAs, and epigenetics. Int J Mol Sci 22:117
Zhou ZH, Wang Y, Ye XY, Li ZG (2018) Signaling molecule hydrogen sulfide improves seed germination and seedling growth of maize (Zea mays L.) under high temperature by inducing antioxidant system and osmolyte biosynthesis. Front Plant Sci 9:1288
Funding
This study is supported by National Natural Science Foundation of China (31760069). In addition, we are grateful to Guangzhou Genedenovo Biotechnology Co., Ltd. for assisting in sequencing and/or bioinformatics analysis.
Author information
Authors and Affiliations
Contributions
ZGL conceived and designed experiments and wrote the manuscript; XYY performed experiments. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Handling Editor: Néstor Carrillo.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, ZG., Ye, XY. Transcriptome response of maize (Zea mays L.) seedlings to heat stress. Protoplasma 259, 357–369 (2022). https://doi.org/10.1007/s00709-021-01680-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-021-01680-8