Abstract
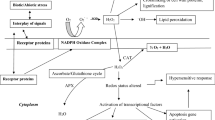
The enhanced generation of reactive oxygen species (ROS) under metal/metalloid stress is most common in plants, and the elevated ROS must be successfully metabolized in order to maintain plant growth, development, and productivity. Ascorbate (AsA) is a highly abundant metabolite and a water-soluble antioxidant, which besides positively influencing various aspects in plants acts also as an enigmatic component of plant defense armory. As a significant component of the ascorbate-glutathione (AsA-GSH) pathway, it performs multiple vital functions in plants including growth and development by either directly or indirectly metabolizing ROS and its products. Enzymes such as monodehydroascorbate reductase (MDHAR, EC 1.6.5.4) and dehydroascorbate reductase (DHAR, EC 1.8.5.1) maintain the reduced form of AsA pool besides metabolically controlling the ratio of AsA with its oxidized form (dehydroascorbate, DHA). Ascorbate peroxidase (APX, EC 1.11.1.11) utilizes the reduced AsA pool as the specific electron donor during ROS metabolism. Thus, AsA, its redox couple (AsA/DHA), and related enzymes (MDHAR, DHAR, and APX) cumulatively form an AsA redox system to efficiently protect plants particularly against potential anomalies caused by ROS and its products. Here we present a critical assessment of the recent research reports available on metal/metalloid-accrued modulation of reduced AsA pool, AsA/DHA redox couple and AsA-related major enzymes, and the cumulative significance of these antioxidant system components in plant metal/metalloid stress tolerance.



Similar content being viewed by others
References
Agius F, Gonzalez-Lamothe R, Caballero JL, Munoz-Blanco J, Botella MA, Valpuesta V (2003) Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat Biotechnol 21:177–181
Aksoy M, Dİnle BS (2012) Changes in physiological parameters and some antioxidant enzymes activities of soybean (Glycine max L. Merr.) leaves under cadmium and salt stress. J Stress Physiol Biochem 8:179–190
Amako K, Chen GX, Asada K (1994) Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol 35:497–504
Anjum NA, Umar S, Ahmad A, Iqbal M (2008) Responses of components of antioxidant system in moongbean (Vigna radiata (L.) Wilczek) genotypes to cadmium stress. Commun Soil Sci Plant Anal 39:2469–2483
Anjum NA, Umar S, Chan MT (eds) (2010) Ascorbate-glutathione pathway and stress tolerance in plants. Springer, Dordrecht
Anjum NA, Umar S, Iqbal M, Khan NA (2011) Cadmium causes oxidative stress in mung mean by affecting the antioxidant enzyme system and ascorbate-glutathione cycle metabolism. Russ J Plant Physiol 58:92–99
Anjum NA, Ahmad I, Mohmood I, Pacheco M, Duarte AC, Pereira E, Umar S, Ahmad A, Khan NA, Iqbal M, Prasad MNV (2012a) Modulation of glutathione and its related enzymes in plants’ responses to toxic metals and metalloids—a review. Environ Exp Bot 75:307–324
Anjum NA, Umar S, Ahmad A (2012b) Oxidative stress in plants: causes, consequences and tolerance. IK International Publishing House, New Delhi
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Aravind P, Prasad MNV (2005) Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate-glutathione cycle and glutathione metabolism. Plant Physiol Biochem 43:107–116
Arrigoni O (1994) Ascorbate system in plant development. J Bioenerg Biomembr 26:407–419
Arrigoni O, De Tullio MC (2000) The role of ascorbic acid in cell metabolism: between gene-directed functions and unpredictable chemical reactions. J Plant Physiol 157:481–488
Arrigoni O, De Tullio MC (2002) Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta 1569:1–9
Arrigoni O, Calabrese G, De Gara L, Bitonti MB, Liso R (1997) Correlation between changes in cell ascorbate and growth of Lupinus albus seedlings. J Plant Physiol 150:302–308
Asada K (1992) Ascorbate peroxidase—a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant 85:235–241
Asada K (1993) Divergence of peroxide-scavenging peroxidases in organisms. In: Yagi K (ed) Active oxygens, lipid peroxides and antioxidants. CRC, Boca Raton, pp 289–298
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Asada K, Takahashi M (1987) Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Bowler CB, Arntzen CB (eds) Photoinhibition. Elsevier, Amsterdam, pp 227–288
Asard H, Horemans N, Caubergs RJ (1995) Involvement of ascorbic acid and a b-type cytochrome in plant plasma membrane redox reactions. Protoplasma 184:36–41
Badejo AA, Fujikawa Y, Esaka M (2009) Gene expression of ascorbic acid biosynthesis related enzymes of the Smirnoff-Wheeler pathway in acerola (Malpighia glabra). J Plant Physiol 166:652–660
Balestrasse KB, Gardey L, Gallego SM, Tomaro ML (2001) Response of antioxidant defence system in soybean nodules and roots subjected to cadmium stress. Aust J Plant Physiol 28:497–504
Barth C, De Tullio M, Conklin PL (2006) The role of ascorbic acid in the control of flowering time and the onset of senescence. J Exp Bot 57:1657–1665
Bartoli CG, Pastori GM, Foyer CH (2000) Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol 123:335–343
Borracino G, Dipierro S, Arrigoni O (1986) Purification and properties of ascorbate free radical reductase from potato tubers. Planta 167:521–526
Boveris A, Sies H, Martino EE, Docampo R, Turrens JF, Stoppani AO (1980) Deficient metabolic utilization of hydrogen peroxide in Trypanosoma cruzi. Biochem J 188:643–648
Burkey KO, Eason G, Fiscus EL (2003) Factors that affect leaf extracellular ascorbic acid content and redox status. Physiol Plant 117:51–57
Candan N, Tarhan L (2003) The correlation between antioxidant enzyme activities and lipid peroxidation levels in Mentha pulegium organs grown in Ca2+, Mg2+, Cu2+, Zn2+ and Mn2+ stress conditions. Plant Sci 165:769–776
Carlos G, Pastori GM, Foyer CH (2000) Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol 123:335–343
Caverzan A, Passaia G, Rosa SB, Ribeiro CW, Lazzarotto F, Margis-Pinheiro M (2012) Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genet Mol Biol 35:1011–1019
Chao YY, Hong CY, Kao CH (2010) The decline in ascorbic acid content is associated with cadmium toxicity of rice seedlings. Plant Physiol Biochem 48:374–381
Chen Z, Gallie DR (2006) Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol 142:775–787
Chen Z, Young TE, Ling J, Chang SC, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci U S A 100:3525–3530
Chen F, Wang F, Wu F, Mao W, Zhang G, Zhou M (2010) Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol Biochem 48:663–672
Chew O, Whelan J, Millar AH (2003) Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J Biol Chem 278:46869–46877
Conklin PL, Barth C (2004) Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ 27:959–970
Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL (1999) Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc Natl Acad Sci U S A 96:4198–203
Córdoba F, Gonzales-Reyes JA (1994) Ascorbate and plant cell growth. J Bioenerg Biomembr 26:399–405
Córdoba-Pedregosa MC, Gonzalez-Reyes JA, Canadillas MS, Navas P, Cordoba F (1996) Role of apoplastic and cell-wall peroxidases on the stimulation of root elongation by ascorbate. Plant Physiol 112:1119–1125
Córdoba-Pedregosa MC, Córdoba F, Villalba JM, José Antonio González-Reyes JA (2003) Zonal changes in ascorbate and hydrogen peroxide contents, peroxidase, and ascorbate-related enzyme activities in onion roots. Plant Physiol 131:697–706
Cuypers A, Vangronsveld J, Clijsters H (2000) Biphasic effect of copper on the ascorbate-glutathione pathway in primary leaves of Phaseolus vulgaris seedlings during the early stages of metal assimilation. Physiol Plant 110:512–517
Cuypers A, Remans T, Weyens N, Colpaert J, Vassilev A, Vangronsveld J (2013) Soil-plant relationships of heavy metals and metalloids. In: Alloway BJ (ed) Heavy metals in soils. Springer, Dordrecht, pp 161–193
Dabrowska G, Kata A, Goc A, Szechynska-Hebda M, Skrzypek E (2007) Characteristics of the plant ascorbate peroxidase family. Acta Biol 49:7–17
Dalton DA, Langeberg L, Robbins M (1992) Purification and characterization of monodehydroascorbate reductase from soybean root nodules. Arch Biochem Biophys 292:281–286
Davey MW, Van MM, Inze D, Sanmartin M, Kanellis A, Smirnoff N et al (2000) Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J Sci Food Agric 80:825–860
De Gara L, Locato V, Dipierro S, de Pinto MC (2010) Redox homeostasis in plants. The challenge of living with endogenous oxygen production. Resp Physiol Neurobiol 173(Suppl):S13–S19
De Leonardis S, De Lorenzo G, Borraccino G, Dipierro S (1995) A specific ascorbate free radical reductase isozyme participates in the regeneration of ascorbate for scavenging toxic oxygen species in potato tuber mitochondria. Plant Physiol 109:847–851
De Leonardis S, Dipierro N, Dipierro S (2000) Purification and characterization of an ascorbate peroxidase from potato tuber mitochondria. Plant Physiol Biochem 38:773–779
de Pinto MC, De Gara L (2004) Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell differentiation. J Exp Bot 55:2559–2569
de Pinto MC, Francis D, De Gara L (1999) The redox state of the ascorbate-dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma 209:90–97
de Pinto MC, Paradiso A, Leonetti P, De Gara L (2006) Hydrogen peroxide, nitric oxide and cytosolic ascorbate peroxidase at the crossroad between defence and cell death. Plant J 48:784–795
Debolt S, Melino V, Ford CM (2007) Ascorbate as a biosynthetic precursor in plants. Ann Bot 99:3–8
Deleonardis S, Delorenzo G, Borraccino G, Dipierro S (1995) A specific ascorbate free radical reductase isozyme participates in the regeneration of ascorbate for scavenging toxic oxygen species in potato tuber mitochondria. Plant Physiol 109:847–851
Dipierro N, Mondelli D, Paciolla C, Brunetti G, Dipierro S (2005) Changes in the ascorbate system in the response of pumpkin (Cucurbita pepo L.) roots to aluminium stress. J Plant Physiol 162:529–536
Diwan H, Ahmad A, Iqbal M (2008) Genotypic variation in the phytoremediation potential of Indian mustard for chromium. J Environ Manage 41:734–741
Dong JG, Fernández-Maculet JC, Yang SF (1992) Purification and characterization of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proc Natl Acad Sci U S A 89:9789–9793
Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N (2007) Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J 52:673–689
Drew DP, Lunde C, Lahnstein J, Fincher GVB (2007) Heterologous expression of cDNAs encoding monodehydroascorbate reductases from the moss, Physcomitrella patens and characterization of the expressed enzymes. Planta 225:945–954
Eastmond PJ (2007) MONODEHYROASCORBATE REDUCTASE4 is required for seed storage oil hydrolysis and postgerminative growth in Arabidopsis. Plant Cell 19:1376–1387
Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Shibahara T, Inanaga S, Tanaka K (2007) Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 225:1255–1264
Fecht-Christoffers MM, Maier P, Horst WJ (2003) Apoplastic peroxidases and ascorbate are involved in manganese toxicity and tolerance of Vigna unguiculata. Physiol Plant 117:237–244
Fiorani M, De Sanctis R, Scarlatti F, Vallorani L, De Bellis R, Serafini G, Bianchi M, Stocchi V (2000) Dehydroascorbic acid irreversibly inhibits hexokinase activity. Mol Cell Biochem 209:145–153
Fotopoulos V, Ziogas V, Tanou G, Molassiotis A (2010) Involvement of AsA/DHA and GSH/GSSG ratios in gene and protein expression and in the activation of defence mechanisms under abiotic stress conditions. In: Anjum NA, Umar S, Chan MT (eds) Ascorbate-glutathione pathway and stress tolerance in plants. Springer, Dordrecht, pp 265–302
Foyer CH (1993) Ascorbic acid. In: Alscher RG, Hess JL (eds) Antioxidants in higher plants. CRC, Boca Raton, pp 31–58
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Foyer CH, Halliwell B (1977) Purification and properties of dehydroascorbate reductase from spinach leaves on the growth of hybrid poplar. Plant Dis 66:587–589
Foyer CH, Mullineaux PM (1998) The presence of dehydroascorbate and dehydroascorbate reductase in plant tissues. FEBS Lett 425:528–529
Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119:355–364
Foyer CH, Noctor G (2009) Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal 11:861–905
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155:93–100
Frei M, Wissuwa M, Pariasca-Tanaka J, Chen CP, Südekum K-H, Kohno Y (2012) Leaf ascorbic acid level—is it really important for ozone tolerance in rice? Plant Physiol Biochem 59:63–70
Gajewska E, Sklodowska M (2008) Differential biochemical responses of wheat shoots and roots to nickel stress: antioxidative reactions and proline accumulation. Plant Growth Regul 54:179–188
Gatzek S, Wheeler GL, Smirnoff N (2002) Antisense suppression of L-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated L-galactose synthesis. Plant J 30:541–553
Gest N, Gautier H, Stevens R (2012) Ascorbate as seen through plant evolution: the rise of a successful molecule? J Exp Bot 64:33–53
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Hasanuzzaman M, Fujita M (2011) Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res 143:1758–1776
Hasanuzzaman M, Fujita M (2013) Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicol 22:584–596
Hasanuzzaman M, Hossain MA, Fujita M (2010) Physiological and biochemical mechanisms of nitric oxide induced abiotic stress tolerance in plants. Am J Plant Physiol 5:295–324
Hasanuzzaman M, Hossain MA, Fujita M (2011a) Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol Rep 5:353–365
Hasanuzzaman M, Hossain MA, Fujita M (2011b) Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol Trace Elem Res 143:1704–1721
Hasanuzzaman M, Hossain MA, Fujita M (2012a) Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating the antioxidant defense and methylglyoxal detoxification systems. Biol Trace Elem Res 149:248–261
Hasanuzzaman M, Hossain MA, Teixeira da Silva JA, Fujita M (2012b) Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. In: Venkateshwarulu B, Shanker AK, Shanker C, Mandapaka M (eds) Crop stress and its management: perspectives and strategies. Springer, Berlin, pp 261–316
Hasanuzzaman M, Nahar K, Alam MM, Fujita M (2012c) Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticum aestivum) seedlings by modulating the antioxidant defense and glyoxalase system. Aust J Crop Sci 6:1314–1323
Hatata MM, Abdel-Aal EA (2008) Oxidative stress and antioxidant defence mechanisms in response to cadmium treatments. Am Eurasian J Agric Environ Sci 4:655–669
Hegedus A, Erdei S, Horvath G (2001) Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress. Plant Sci 160:1085–1093
Herschbach C, Scheerer U, Rennenberg H (2010) Redox states of glutathione and ascorbate in root tips of poplar (Populus tremula × P. alba) depend on phloem transport from the shoot to the roots. J Exp Bot 61:1065–1074
Horemans N, Asard H, Caubergs RJ (1997) The ascorbate carrier of higher plant plasma membranes preferentially translocates the fully oxidized (dehydroascorbate) molecule. Plant Physiol 114:1247–1253
Horemans N, Asard H, Caubergs RJ (1998) Carrier mediated uptake of dehydroascorbate into higher plant plasma membrane vesicles shows trans-stimulation. FEBS Lett 421:41–44
Horemans N, Foyer CH, Asard H (2000) Transport and action of ascorbate at the plant plasma membrane. Trend Plant Sci 5:263–267
Horemans N, Raeymaekers T, Beek KV, Nowocin A, Blust R, Broos K, Cuypers A et al (2007) Dehydroascorbate uptake is impaired in the early response of Arabidopsis plant cell cultures to cadmium. J Exp Bot 58:4307–4317
Hossain MA, Asada K (1985) Monodehydroascorbate reductase from cucumber is a flavin adenine dinucleotide enzyme. J Biol Chem 260:12920–12926
Hossain MA, Fujita M (2012) Regulatory role of components of ascorbate-glutathione (AsA-GSH) pathway in plant tolerance to oxidative stress. In: Anjum NA, Umar S, Ahmed A (eds) Oxidative stress in plants: causes, consequences and tolerance. IK International Publishing House, New Delhi, pp 81–147
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395
Hossain MA, Hasanuzzaman M, Fujita M (2010) Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Plant 16:259–272
Imai T, Karita S, Shiratori G, Hattori M, Nunome T, Oba K et al (1998) L-Galactono-gamma-lactone dehydrogenase from sweet potato: purification and cDNA sequence analysis. Plant Cell Physiol 39:1350–1358
Iqbal N, Masood A, Nazar R, Syeed S, Khan NA (2010) Photosynthesis, growth and antioxidant metabolism in mustard (Brassica juncea L.) cultivars differing in cadmium tolerance. Agric Sci China 9:519–527
Ishikawa T, Shigeoka S (2008) Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci Biotechnol Biochem 72:1143–1154
Ishikawa T, Yoshimura K, Sakai K, Tamoi M, Takeda T, Shigeoka S (1998) Molecular characterization and physiological role of a glyoxysome-bound ascorbate peroxidase from spinach. Plant Cell Physiol 39:23–34
Ishikawa T, Dowdle J, Smirnoff N (2006) Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiol Plant 126:343–355
Islam MM, Hoque MA, Okuma E, Jannat R, Banu MNA, Jahan MS et al (2009) Proline and glycinebetaine confer cadmium tolerance on tobacco bright yellow-2 cells by increasing ascorbate-glutathione cycle enzyme activities. Biosci Biotechnol Biochem 73:2320–2323
Israr M, Sahi SV, Jain J (2006) Cadmium accumulation and antioxidative responses in the Sesbania drumondiiallus. Arch Environ Contam Toxicol 50:121–127
Israr M, Jewell A, Kumar D, Sahi SV (2011) Interactive effects of lead, copper, nickel and zinc on growth, metal uptake and antioxidative metabolism of Sesbania drummondii. J Hazard Mater 186:1520–1526
Jiménez A, Hernández JA, Del Rio LA, Sevilla F (1997) Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114:275–284
Jin X, Yang X, Mahmood Q, Islam E, Liu D, Li H (2008) Response of antioxidant enzymes, ascorbate and glutathione metabolism towards cadmium in hyperaccumulator and nonhyperaccumulator ecotypes of Sedum alfredii H. Environ Toxicol 23:517–529
Justi KC, Visentainer JV, deSouza NE, Matsushita M (2000) Nutritional composition and vitamin C stability in stored camu-camu (Myrciaria dubia) pulp. Arch Latinoam Nutr 50:405–408
Kanwar MK, Bhardwaj R, Arora P, Chowdhary SP, Sharma P, Kumar S (2012) Plant steroid hormones produced under Ni stress are involved in the regulation of metal uptake and oxidative stress in Brassica juncea L. Chemosphere 86:41–49
Karuppanapandian T, Manoharan K (2008) Uptake and translocation of tri- and hexa-valent chromium and their effects on black gram (Vigna mungo L. Hepper cv. Co4) Roots. J Plant Biol 51:192–201
Karyotou K, Donaldson RP (2005) Ascorbate peroxidase, a scavenger of hydrogen peroxide in glyoxysomal membranes. Arch Biochem Biophys 434:248–257
Kato N, Esaka M (1999) Changes in ascorbate oxidase gene expression and ascorbate levels in cell division and cell elongation in tobacco cells. Physiol Plant 105:321–329
Khan NA, Samiullah SS, Nazar R (2007) Activities of antioxidative enzymes, sulphur assimilation, photosynthetic activity and growth of wheat (Triticum aestivum) cultivars differing in yield potential under cadmium stress. J Agron Crop Sci 193:435–444
Khan I, Ahmad A, Iqbal M (2009) Modulation of antioxidant defence system for arsenic detoxification in Indian mustard. Ecotoxicol Environ Saf 72:626–634
Kieselbach T, Bystedt M, Hynds P, Robinson C, Schröder WP (2000) A peroxidase homologue and novel plastocyanin located by proteomics to the Arabidopsis chloroplast thylakoid lumen. FEBS Lett 480:271–276
Kotchoni SO, Larrimore KE, Mukherjee M, Kempinski CF, Barth C (2009) Alterations in the endogenous ascorbic acid content affect flowering time in Arabidopsis. Plant Physiol 149:803–815
Krämer U (2010) Metal hyperaccumulation in plants. Annu Rev Plant Biol 61:517–534
Krantev A, Yordanova R, Janda T, Szalai G, Popova L (2008) Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol 165:920–931
Kwon SY, Choi SM, Ahn YO, Lee HS, Lee HB, Park YM, Kwak SS (2003) Enhanced stress-tolerance of transgenic tobacco plants expressing a human dehydroascorbate reductase gene. J Plant Physiol 160:347–353
Laing WA, Bulley S, Wright M, Cooney J, Jensen D, Barraclough D, MacRae E (2004) A highly specific L-galactose-1-phosphate phosphatase on the path to ascorbate biosynthesis. Proc Natl Acad Sci U S A 101:16976–16981
Laing WA, Wright MA, Cooney J, Bulley SM (2007) The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an L-galactose guanyltransferase, increases leaf ascorbate content. Proc Natl Acad Sci U S A 104:9534–9539
Lamhamdi M, Bakrim A, Aarab A, Lafont R, Sayah F (2011) Effects of lead phytotoxicity on wheat (Triticum aestivum L.) seed germination and seedling growth. C R Biol 334:118–126
Lee YP, Kim SH, Bang JW, Lee HS, Kwak SS, Kwon SY (2007) Enhanced tolerance to oxidative stress in transgenic tobacco plants expressing three antioxidant enzymes in chloroplasts. Plant Cell Rep 26:591–598
Leterrier M, Corpas FJ, Barroso JB, Sandalio LM, del Rio LA (2005) Peroxisomal monodehydroascorbate reductase. Genomic clone characterization and functional analysis under environmental stress conditions. Plant Physiol 138:2111–2123
Li M, Ma F, Guo C, Liu J (2010) Ascorbic acid formation and profiling of genes expressed in its synthesis and recycling in apple leaves of different ages. Plant Physiol Biochem 48:216–224
Li X, Ma H, Jia P, Wang J, Jia L, Zhang T, Yang Y, Chen H, Wei X (2012) Responses of seedling growth and antioxidant activity to excess iron and copper in Triticum aestivum L. Ecotoxicol Environ Saf 86:47–53
Linster CL, Clarke SG (2008) L-Ascorbate biosynthesis in higher plants: the role of VTC2. Trend Plant Sci 13:567–573
Liso R, Innocenti AM, Bitonti MB, Arrigoni O (1988) Ascorbic acid-induced progression of quiescent center cells from G1 to S phase. New Phytol 110:469–471
Liso R, De Tullio MC, Ciraci S, Balestrini R, La Rocca N et al (2004) Localisation of ascorbic acid, ascorbic acid oxidase, and glutathione in roots of Cucurbita maxima L. J Exp Bot 55:2589–2597
Liu X, Shiomi S, Nakatsuka A, Kubo Y, Nakamura R, Inaba A (1999) Characterization of ethylene biosynthesis associated with ripening in banana fruit. Plant Physiol 121:1257–1266
Locato V, de Pinto MC, De Gara L (2009) Different involvement of the mitochondrial, plastidal and cytosolic ascorbate-glutathione redox enzymes in heat shock responses. Phyiol Plant 135:296–306
Loewus FA (1999) Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry 52:193–210
Lopéz-Carbonell M, Munné-Bosch S, Alegre L (2006) The ascorbate-deficient vtc-1 Arabidopsis mutant shows altered ABA accumulation in leaves and chloroplast. J Plant Growth Regul 25:137–144
Lorence A, Chevone BI, Mendes P, Nessler CL (2004) myo-Inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol 134:1200–1205
Lunde C, Baumann U, Shirley NJ, Drew DP, Fincher GB (2006) Gene structure and expression pattern analysis of three monodehydroascorbate reductase (Mdhar) genes in Physcomitrella patens: implications for the evolution of the MDHAR family in plants. Plant Mol Biol 60:259–275
Lyubenova L, Schröder P (2011) Plants for waste water treatment—effects of heavy metals on the detoxification system of Typha latifolia. Bioresource Technol 102:996–1004
Maheshwari R, Dubey RS (2009) Nickel-induced oxidative stress and the role of antioxidative defense in rice seedlings. Plant Growth Regul 59:37–49
Maksymiec W (2007) Signalling responses in plants to heavy metal stress. Acta Physiol Plant 29:177–187
Mano J, Hideg E, Asada K (2004) Ascorbate in thylakoid lumen functions as an alternative electron donor to photosystem II and photosystem I. Arch Biochem Biophys 429:71–80
Markovska YK, Gorinova NI, Nedkovska MP, Miteva KM (2009) Cadmium-induced oxidative damage and antioxidant responses in Brassica juncea plants. Biol Plant 53:151–154
Martínez JP, Araya H (2010) Ascorbate-glutathione cycle: enzymatic and non-enzymatic integrated mechanisms and its biomolecular regulation. In: Anjum NA, Chan MT, Umar S (eds) Ascorbate-glutathione pathway and stress tolerance in plants. Springer, Dordrecht, pp 303–322
Mathews MC, Summers CB, Felton GW (1997) Ascorbate peroxidase: a novel antioxidant enzyme in insects. Arch Insect Biochem Physiol 34:57–68
Mishra S, Jha AB, Dubey RS (2011) Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma 248:565–577
Mittler R, Zilinskas BA (1991) Purification and characterization of pea cytosolic ascorbate peroxidase. Plant Physiol 97:962–968
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trend Plant Sci 9:490–498
Mittova V, Tal M, Volokita M, Guy M (2003) Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ 26:845–856
Miyake C, Asada K (1992) Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol 33:541–553
Miyake C, Asada K (1994) Ferredoxin-dependent photoreduction of the monodehydroascorbate radical in spinach thylakoids. Plant Cell Physiol 35:539–549
Miyake C, Michihata F, Asada K (1991) Scavenging of hydrogen peroxide in prokaryotic and eukaryotic algae: acquisition of ascorbate peroxidase during the evolution of cyanobacteria. Plant Cell Physiol 32:33–43
Mobin M, Khan NA (2007) Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164:601–610
Mohamed AA, Castagna A, Ranieri A, Sanità di Toppi L (2012) Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol Biochem 57C:15–22
Morell S, Follmann H, De Tullio M, Häberlein I (1997) Dehydroascorbate and dehydroascorbate reductase are phantom indicators of oxidative stress in plants. FEBS Lett 414:567–570
Mullen RT, Trelease RN (1996) Biogenesis and membrane properties of peroxisomes: does the boundary membrane serve and protect? Trend Plant Sci 1:389–394
Mullineaux P, Karpinski S (2002) Signal transduction in response to excess light: getting out of the chloroplast. Curr Opin Plant Biol 5:43–48
Murthy SS, Zilinskas BA (1994) Molecular cloning and characterization of a DNA encoding pea monodehydroascorbate reductase. J Biol Chem 269:31129–31133
Nehnevajova E, Lyubenova L, Herzig R, Schröder P, Schwitzguébel JP, Schmülling T (2012) Metal accumulation and response of antioxidant enzymes in seedlings and adult sunflower mutants with improved metal removal traits on a metal-contaminated soil. Environ Exp Bot 76:39–48
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Noctor G, Arisi ACM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH (1998) Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot 49:623–647
Nouairi I, Ammar WB, Youssef NB, Miled DDB, Ghorbal MH, Zarrouk M (2009) Antioxidant defense system in leaves of Indian mustard (Brassica juncea) and rape (Brassica napus) under cadmium stress. Acta Physiol Plant 31:237–247
Paciolla C, De Tullio MC, Chiappetta A, Innocenti AM, Bitonti MB, Liso R, Arrigoni O (2001) Short- and long-term effects of dehydroascorbate in Lupinus albus and Allium cepa roots. Plant Cell Physiol 42:857–863
Pang CH, Wang BS (2010) Role of ascorbate peroxidase and glutathione reductase in ascorbate-glutathione cycle and stress tolerance in plants. In: Anjum NA, Umar S, Chan MT (eds) Ascorbate-glutathione pathway and stress tolerance in plants. Springer, Dordrecht, pp 91–112
Paradiso A, Berardino R, De Pinto MC et al (2008) Increase in ascorbate-glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol 49:362–374
Parsons HT, Fry SC (2012) Oxidation of dehydroascorbic acid and 2,3-diketogulonate under plant apoplastic conditions. Phytochemistry 75:41–49
Parsons HT, Yasmin T, Fry SC (2011) Alternative pathways of dehydroascorbic acid degradation in vitro and in plant cell cultures: novel insights into vitamin C catabolism. Biochem J 440:375–383
Pastori GM, Foyer CH (2002) Common components, networks and pathways of cross tolerance to stress: the central role of ‘redox’ and ABA-mediated controls. Plant Physiol 129:460–468
Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S et al (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell Online 15:939–951
Pekker I, Tel-Or E, Mittler R (2002) Reactive oxygen intermediates and glutathione regulate the expression of cytosolic ascorbate peroxidase during iron-mediated oxidative stress in bean. Plant Mol Biol 49:429–438
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. doi:10.1155/2012/217037
Pignocchi C, Kiddle G, Hernández I, Foster SJ, Asensi A et al (2006) Ascorbate oxidase-dependent changes in the redox state of the apoplast modulate gene transcript accumulation leading to modified hormone signaling and orchestration of defense processes in tobacco. Plant Physiol 141:423–435
Polle A (2001) Dissecting the superoxide dismutase-ascorbate-glutathione-pathway in chloroplasts by metabolic modeling—computer simulations as a step towards flux analysis. Plant Physiol 126:445–462
Posmyk MM, Kontek R, Janas KM (2009) Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings exposed to copper stress. Ecotoxicol Environ Saf 72:596–602
Potters G, Horemans N, Caubergs RJ, Asard H (2000) Ascorbate and dehydroascorbate influence cell cycle progression in tobacco cell suspension. Plant Physiol 124:17–20
Potters G, De Gara L, Asard H, Horemans N (2002) Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiol Biochem 40:537–548
Potters G, Horemans N, Bellone S, Caubergs RJ, Trost P, Guisez Y, Asard H (2004) Dehydroascorbate influences the plant cell cycle through a glutathione-independent reduction mechanism. Plant Physiol 134:1479–1487
Potters G, Horemans N, Jansen MAK (2010) The cellular redox state in plant stress biology—a charging concept. Plant Physiol Biochem 48:292–300
Pourcel L, Routaboul JM, Cheynier V (2007) Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trend Plant Sci 12:29–36
Prasad MNV (2004) Heavy metal stress in plants: from biomolecules to ecosystems, 2nd edn. Springer, Berlin
Qureshi MI, Israr M, Abdin MZ, Iqbal M (2005) Responses of Artemisia annua L. to lead and salt-induced oxidative stress. Environ Exp Bot 53:185–193
Rizhsky L, Hallak-Herr E, Van Breusegem F, Rachmilevitch S, Barr JE et al (2002) Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J 32:329–342
Romero-Puertas MC, Corpas FJ, Rodriguez-Serrano M, Gomez M et al (2007) Differential expression and regulation of antioxidative enzymes by cadmium in pea plants. J Plant Physiol 164:1346–1357
Rosa SB, Caverzan A, Teixeira FK, Lazzarotto F, Silveira JA et al (2010) Cytosolic APx knockdown indicates an ambiguous redox responses in rice. Phytochemistry 71:548–558
Saeidi-Sar S, Khavari-Nejad RA, Fahimi H, Ghorbanli M, Majd A (2007) Interactive effects of gibberellin A3 and ascorbic acid on lipid peroxidation and antioxidant enzyme activities in Glycine max seedlings under nickel stress. Russ J Plant Physiol 54:74–79
Sakihama Y, Mano JI, Sano S, Asada K, Yamasaki H (2000) Reduction of phenoxyl radicals mediated by monodehydroascorbate reductase. Biochem Biophys Res Commun 279:949–954
Sanmartin M, Drogoudi P, Lyons T, Pateraki I, Barnes J, Kanellis A (2003) Overexpression of ascorbate oxidase in the apoplast of transgenic tobacco results in altered ascorbate and glutathione redox states and increased sensitivity to ozone. Planta 216:918–928
Sano S, Ueda M, Kitajima S, Takeda T, Shigeoka S et al (2001) Characterization of ascorbate peroxidases from unicellular red alga Galdieria partita. Plant Cell Physiol 42:433–440
Sano S, Tao S, Endo Y, Inaba T, Hossain MA et al (2005) Purification and cDNA cloning of chloroplastic monodehydroascorbate reductase from spinach. Biosci Biotechnol Biochem 69:762–772
Scandalios JG (2002) The rise of ROS. Trends Biochem Sci 27:483–486
Schröder P, Lyudmila L, Huber C (2009) Do heavy metals and metalloids influence the detoxification of organic xenobiotics in plants? Environ Sci Pollut Res 16:795–804
Schützendübel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R et al (2001) Cadmium-induced changes in antioxidative systems, H2O2 content and differentiation in pine (Pinus sylvestris) roots. Plant Physiol 127:887–898
Schützendübel A, Nikolova P, Rudolf C, Polle A (2002) Cadmium and H2O2 induced oxidative stress in Populas × canescens roots. Plant Physiol Biochem 40:577–584
Sharma P, Dubey RS (2007) Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminium. Plant Cell Rep 26:2027–2038
Shen GM, Zhu C, Shangguan LN, Du QZ (2012) The Cd-tolerant rice mutant cadH-5 is a high Cd accumulator and shows enhanced antioxidant activity. J Plant Nutr Soil Sci 175:309–318
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53:1305–1319
Shimaoka T, Yokota A, Miyake C (2000) Purification and characterization of chloroplast dehydroascorbate reductase from spinach leaves. Plant Cell Physiol 41:1110–1118
Siendones E, Gonzales-Reyes JA, Santos-Ocana C, Navas P, Cordoba F (1999) Biosynthesis of ascorbic acid in kidney bean. L-galactono-γ-lactone dehydrogenase is an intrinsic protein located at the mitochondrial inner membrane. Plant Physiol 120:907–912
Singh S, Khan NA, Nazar R, Anjum NA (2008) Photosynthetic traits and activities of antioxidant enzymes in blackgram (Vigna mungo L. Hepper) under cadmium stress. Am J Plant Physiol 3:25–32
Singh R, Gautam N, Mishra A, Gupta R (2011) Heavy metals and living systems: an overview. Indian J Pharmacol 43:246–53
Singh VP, Srivastava PK, Prasad SM (2013) Nitric oxide alleviates arsenic-induced toxic effects in ridged Luffa seedlings. Plant Physiol Biochem 71:155–163
Smirnoff N (1996) The function and metabolism of ascorbic acid in plants. Ann Bot 78:661–669
Smirnoff N (2000) Ascorbate biosynthesis and function in photoprotection. PhilTrans Royal Soc London B 355:1455–1464
Smirnoff N (2011) Vitamin C: the metabolism and functions of ascorbic acid in plants. Adv Bot Res 59:107–177
Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Critic Rev Biochem Mol Biol 35:291–314
Smirnoff N, Conklin PL, Loewus FA (2001) Biosynthesis of ascorbic acids in plants: a renaissance. Annu Rev Plant Physiol Plant Mol Biol 52:437–467
Sobrino-Plata J, Ortega-Villasante C, Flores-Caceres ML, Escobar C, Del Campo FF, Hernandez LE (2009) Differential alterations of antioxidant defenses as bioindicators of mercury and cadmium toxicity in alfalfa. Chemosphere 77:946–954
Sytar O, Kumar A, Latowski D, Kuczynska P, Strzałka K, Prasad MNV (2013) Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol Plant 35:985–999
Takahama U, Oniki T (1992) Regulation of peroxidase-dependent oxidation of phenolics in the apoplast of spinach leaves by ascorbate. Plant Cell Physiol 33:379–387
Takeda T, Yoshimura K, Ishikawa T, Shigeoka S (1998) Purification and characterization of ascorbate peroxidase in Chlorella vulgaris. Biochimie 80:295–301
Talukdar T, Talukdar D (2013) Response of antioxidative enzymes to arsenic-induced phytotoxicity in leaves of a medicinal daisy, Wedelia chinensis Merrill. J Nat Sci Biol Med 4:383–388
Teixeira FK, Menezes-Benavente L, Galvão VC, Margis R, Margis-Pinheiro M (2006) Rice ascorbate peroxidase gene family encodes functionally diverse isoforms localized in different subcellular compartments. Planta 224:300–314
Tewari RK, Parma PK, Sharma N (2006) Antioxidant responses to enhanced generation of superoxide anion radical and hydrogen peroxide in the copper-stressed mulberry plants. Planta 223:1145–1153
Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma GD et al (2012) Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem 53:33–39
Touiserkani T, Haddad R (2012) Cadmium-induced stress and antioxidative responses in different Brassica napus cultivars. J Agr Sci Tech 14:929–937
Tripathi P, Mishra A, Dwivedi S, Chakrabarty D, Trivedi PK, Singh RP, Tripathi RD (2012) Differential response of oxidative stress and thiol metabolism in contrasting rice genotypes for arsenic tolerance. Ecotoxicol Environ Saf 79:189–198
Wada N, Kinoshita S, Matsuo M, Amako K, Miyake C, Asada K (1998) Purification and molecular properties of ascorbate peroxidase from bovine eye. Biochem Biophys Res Commun 242:256–261
Wang H, Feng T, Peng X, Yan M, Tang X (2009) Up-regulation of chloroplastic antioxidant capacity is involved in alleviation of nickel toxicity of Zea mays L. by exogenous salicylic acid. Ecotoxicol Environ Saf 72:1354–62
Wang Z, Xiao Y, Chen W, Tang K, Zhang L (2010) Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J Integ Plant Biol 52:400–409
Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393:365–369
Wolucka BA, VanMontagu M (2003) GDP-D-mannose 300,500-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J Biol Chem 278:47483–47490
Wu FB, Chen F, Wei K, Zhang GP (2004) Effect of cadmium on free amino acid, glutathione and ascorbic acid concentrations in two barley genotypes (Hordeum vulgare L.) differing in cadmium tolerance. Chemosphere 57:447–454
Yamaguchi K, Mori H, Nishimura M (1995) A novel isoenzyme of ascorbate peroxidase localized on glyoxysomal and leaf peroxisomal membranes in pumpkin. Plant Cell Physiol 36:1157–1162
Yin L, Wang S, Eltayeb AE, Uddin MI, Yamamoto Y et al (2010) Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta 231:609–621
Yoon HS, Lee H, Lee IA, Kim KY, Jo JK (2004) Molecular cloning of the monodehydroascorbate reductase gene from Brassica campestris and analysis of its mRNA level in response to oxidative stress. Biochim Biophys Acta Bioenerg 1658:181–186
Yoshida S, Tamaoki M, Shikano T, Nakajima N, Ogawa D, Ioki M, Aono M, Kubo A, Kamada H, Inoue Y, Saji H (2006) Cytosolic dehydroascorbate reductase is important for ozone tolerance in Arabidopsis thaliana. Plant Cell Physiol 47:304–308
Zelinová V, Bočová B, Huttová J, Mistrík I, Tamás L (2013) Impact of cadmium and hydrogen peroxide on ascorbate-glutathione recycling enzymes in barley root. Plant Soil Environ 59:62–67
Zhang FQ, Shi WY, Jin ZX, Shen ZG (2003) Response of antioxidative enzymes in cucumber chloroplast to cadmium toxicity. J Plant Nutr 26:1779–1788
Zhao ZQ, Cai YL, Zhu YG, Kneer R (2005) Cadmium-induced oxidative stress and protection by L-galactono-1, 4-lactone in winter wheat (Triticum aestivum L.). J Plant Nutr Soil Sci 168:759–763
Acknowledgments
NAA (SFRH/BPD/84671/2012), ACD, EP, and IA are grateful to the Portuguese Foundation for Science and Technology (FCT) and the Aveiro University Research Institute/Centre for Environmental and Marine Studies (CESAM) for partial financial support. SSG, RG, RT and NT acknowledge the funds from CSIR, DST, and UGC, Government of India, New Delhi. The authors apologize if some references related to the main theme of the current article could not be cited due to space constraint.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Handling Editor: David Robinson
Rights and permissions
About this article
Cite this article
Anjum, N.A., Gill, S.S., Gill, R. et al. Metal/metalloid stress tolerance in plants: role of ascorbate, its redox couple, and associated enzymes. Protoplasma 251, 1265–1283 (2014). https://doi.org/10.1007/s00709-014-0636-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0636-x