Abstract
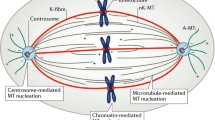
Current spindle models explain “anaphase A” (movement of chromosomes to the poles) in terms of a motility system based solely on microtubules (MTs) and that functions in a manner unique to mitosis. We find both these propositions unlikely. An evolutionary perspective suggests that when the spindle evolved, it should have come to share not only components (e.g., microtubules) of the interphase cell but also the primitive motility systems available, including those using actin and myosin. Other systems also came to be involved in the additional types of motility that now accompany mitosis in extant spindles. The resultant functional redundancy built reliability into this critical and complex process. Such multiple mechanisms are also confusing to those who seek to understand how chromosomes move. Narrowing this commentary down to just anaphase A, we argue that the spindle matrix participates with MTs in anaphase A and that this matrix may contain actin and myosin. The diatom spindle illustrates how such a system could function. This matrix may be motile and work in association with the MT cytoskeleton, as it does with the actin cytoskeleton during cell ruffling and amoeboid movement. Instead of pulling the chromosome polewards, the kinetochore fibre’s role might be to slow polewards movement to allow correct chromosome attachment to the spindle. Perhaps the earliest eukaryotic cell was a cytoplast organised around a radial MT cytoskeleton. For cell division, it separated into two cytoplasts via a spindle of overlapping MTs. Cytokinesis was actin-based cleavage. As chromosomes evolved into individual entities, their interaction with the dividing cytoplast developed into attachment of the kinetochore to radial (cytoplast) MTs. We believe it most likely that cytoplasmic motility systems participated in these events.




Similar content being viewed by others
References
Azoury J, Lee KW, Georget V, Rassinier P, Leader B, Verhlhac M-H (2008) Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr Biol 18:1514–1519. doi:10.1016/j.cub.2008.08.044
Bajer A (1958) Cine-micrographic studies on chromosome movements in ß-irradiated cells. Chromosoma 9:319–331. doi:10.1007/BF02568084
Bajer A (1967) Notes on ultrastructure and some properties of transport within the living mitotic spindle. J Cell Biol 33:713–720. doi:10.1083/jcb.33.3.713
Bucciarelli E, Giansatti MG, Bonaccorsi S, Gatti M (2003) Spindle assembly and cytokinesis in the absence of chromosomes during Drosophila male meiosis. J Cell Biol 160:993–999. doi:10.1083/jcb.200211029
Buster DW, Zhang D, Sharp DJ (2007) Poleward tubulin flux in spindles: regulation and function in mitotic cells. Mol Biol Cell 18:3094–3104. doi:10.1091/mbc.E06-11-0994
Czaban BB, Forer A (1994) Rhodamine-phalloidin and anti-tubulin antibody staining of spindle fibres that were irradiated with an ultraviolet microbeam. Protoplasma 178:18–27. doi:10.1007/BF01404117
Dulyaninova NG, Patskovsky YV, Bresnick AR (2004) The N-terminus of the long MLCK induces a disruption in normal spindle morphology and metaphase arrest. J Cell Sci 117:1481–1493. doi:10.1242/jcs.00993
Fabian L, Forer A (2005) Redundant mechanisms for anaphase chromosome movements: crane-fly spermatocyte spindles normally use actin filaments but also can function without them. Protoplasma 225:169–184. doi:10.1007/s00709-005-0094-6
Fabian L, Forer A (2007) Possible roles of actin and myosin during anaphase chromosome movements in locust spermatocytes. Protoplasma 231:201–213. doi:10.1007/s00709-007-0262-y
Fabian L, Troscianczuk J, Forer A (2007a) Calyculin A, an enhancer of myosin, speeds up anaphase chromosome movement. Cell Chromosome 6:1. Available on line at http://cellandchromosome.com/content/6/1/1.
Fabian L, Xia X, Venkitaramani DV, Johansen KM, Johansen J, Andrew DJ, Forer A (2007b) Titin in insect spermatocyte spindle fibers associates with microtubules, actin, myosin and the matrix proteins skeletor, megator and chromator. J Cell Sci 120:2190–2204. doi:10.1242/jcs.03465
Fishkind DJ, Cao L-G, Wang Y-L (1991) Microinjection of catalytic fragment of myosin light chain kinase into dividing cells: effects on mitosis and cytokinesis. J Cell Biol 114:967–975. doi:10.1083/jcb.114.5.967
Forer A (1988) Do anaphase chromosomes chew their way to the pole or are they pulled by actin? J Cell Sci 91:449–453
Forer A, Pickett-Heaps JD (1998) Cytochalasin D and latrunculin affect chromosome behaviour during meiosis in crane-fly spermatocytes. Chromosome Res 6:533–549. doi:10.1023/A:1009224322399
Forer A, Spurck T, Pickett-Heaps JD, Wilson PJ (2003) Structure of kinetochore fibres in crane-fly spermatocytes after irradiation with and ultraviolet microbeam: neither microtubules nor actin filaments remain in the irradiated region. Cell Motil Cytoskeleton 56:173–192. doi:10.1002/cm.10144
Forer A, Spurck T, Pickett-Heaps JD (2007) Actin and myosin inhibitors block elongation of kinetochore fibre stubs in metaphase crane-fly spermatocytes. Protoplasma 232:79–85. doi:10.1007/s00709-007-0265-8
Forer A, Spurck T, Pickett-Heaps JD (2008) What generates flux of tubulin in kinetochore microtubules? Protoplasma 232:137–141. doi:10.1007/s00709-008-0286-y
Fuge H (1989) Rapid kinetochore movements in Mesostoma ehrenbergii spermatocytes: action of antagonistic chromosome fibres. Cell Motil Cytoskeleton 13:212–220. doi:10.1002/cm.970130308
Gorbsky GJ, Sammak PJ, Borisy GG (1988) Microtubule dynamics and chromosome motion visualized in living anaphase cells. J Cell Biol 106:1185–1192. doi:10.1083/jcb.106.4.1185
Guerriero V Jr, Rowley DR, Means AR (1981) Production and characterization of an antibody to myosin light chain kinase and intracellular localization of the enzyme. Cell 27:449–458. doi:10.1016/0092-8674(81)90386-X
Hughes A (1959) A history of cytology. Abelard-Schuman, London and New York
Inoué S (1964) Organization and function of the mitotic spindle. In: Allen RD, Kamiya N (eds) Primitive motile systems in cell biology. Academic Press, New York London, pp 549–598
Ishigami M, Kuroda K, Hatano S (1987) Dynamic aspects of the contractile system in Physarum plasmodium. III. Cyclic contraction-relaxation of the plasmodial fragment in accordance with the generation-degeneration of cytoplasmic actomyosin fibrils. J Cell Biol 105:381–386. doi:10.1083/jcb.105.1.381
Johansen KM, Johansen J (2007) Cell and molecular biology of the spindle matrix. Int Rev Cytol 26:155–206. doi:10.1016/S0074-7696(07)63004-6
Kamiya N (1968) The mechanism of cytoplasmic movement in a myxomycete plasmodium. Symp Soc Exp Biol 22:199–214
Komatsu S, Yano T, Shibata M, Tuft RA, Ikebe M (2000) Effects of regulatory light chain phosphorylation of myosin II on mitosis and cytokinesis of mammalian cells. J Biol Chem 275:34512–34520. doi:10.1074/jbc.M003019200
Koshland D, Mitchison TM, Kirschner MW (1988) Polewards chromosome movement driven by microtubule depolymerization in vitro. Nature 331:499–504. doi:10.1038/331499a0
La Fountain JR Jr, Oldenbourg R, Cole RW, Rieder CL (2001) Microtubule flux mediates poleward motion of acentric chromosome fragments during meiosis in insect spermatocytes. Mol Biol Cell 12:4054–4065
La Fountain JR Jr, Cole RW, Rieder CL (2002) Polar ejection forces are operative in crane-fly spermatocytes, but their action is limited to the spindle periphery. Cell Motil Cytoskeleton 51:16–26. doi:10.1002/cm.10011
La Fountain JR Jr, Cohan CS, Siegel AJ, La Fountain DJ (2004) Direct visualization of microtubule flux during metaphase and anaphase in crane-fly spermatocytes. Mol Biol Cell 15:5724–5732. doi:10.1091/mbc.E04-08-0750
Lenart P, Bacher CP, Daigle N, Hand AR, Eils R, Terasaki M, Ellenberg J (2005) A contractile nuclear actin network drives chromosomal congression in oocytes. Nature 436:812–818. doi:10.1038/nature03810
Margolis RI, Wilson L, Kiefer BI (1978) Mitotic mechanism based on intrinsic microtubule behaviour. Nature 272:450–452. doi:10.1038/272450a0
Mole-Bajer J, Bajer A, Owczarzak A (1974) Chromosome movements in prometaphase and aster transport in the newt. Cytobios 13:45–65
Nagai R, Yoshimoto Y, Kamiya N (1978) Cyclic production of tension force in the plasmodial strand of Physarum polycephalum and its relation to microfilament morphology. J Cell Sci 33:205–225
Nicklas RB, Koch CA (1972) Chromosome micromanipulation. IV. Polarized motions within the spindle and models for mitosis. Chromosoma 39:1–26. doi:10.1007/BF00320586
Ostergren G, Mole-Bajer J, Bajer A (1960) An interpretation of transport phenomena at mitosis. Ann N Y Acad Sci 90:381–408. doi:10.1111/j.1749-6632.1960.tb23258.x
Pickett-Heaps JD (1974) The evolution of mitosis and the eukaryotic condition. Biosystems 6:37–48. doi:10.1016/0303-2647(74)90009-4
Pickett-Heaps JD (1991) Cell division in diatoms. Int Rev Cytol 123:63–107. doi:10.1016/S0074-7696(08)60497-0
Pickett-Heaps JD, Bajer AJ (1978) Mitosis: an argument for multiple mechanisms achieving chromosomal movement. Cytobios 19:171–180
Pickett-Heaps JD, Carpenter J (1993) An extended corona attached to metaphase kinetochores of the green alga Oedogonium. Eur J Cell Biol 60:300–307
Pickett-Heaps JD, Forer A (2001) Pac-Man does not resolve the enduring problem of anaphase chromosome movement. Protoplasma 215:16–20. doi:10.1007/BF01280300
Pickett-Heaps JD, Pickett-Heaps JF (2002) “The dynamics and mechanics of mitosis”. DVD (NTSC). Available from: cytographics.com
Pickett-Heaps JD, Spurck TP (1982) Studies on kinetochore function in mitosis. I. The effects of colchicine and cytochalasin on mitosis in the diatom Hantzschia amphioxys. Eur J Cell Biol 28:77–82
Pickett-Heaps JD, Forer A, Spurck T (1996) Rethinking anaphase: where “PAC-MAN” fails and why a role for the spindle matrix is likely. Protoplasma 192:1–10. doi:10.1007/BF01273239
Pickett-Heaps JD, Gunning BES, Brown RC, Lemmon BE, Cleary AL (1999) The cytoplast concept in plant cells: cytoplasmic domains and the evolution of spatially organised cell division. Am J Bot 86:153–172. doi:10.2307/2656933
Rebhun LL (1964) Saltatory particle movements in cells. In: Allen RD, Kamiya N (eds) Primitive motile systems in cell biology. Academic Press, New York London, pp 503–525
Rieder CL (2005) Kinetochore fiber formation in animal somatic cells: duelling mechanisms come to a draw. Chromosoma 114:310–318. doi:10.1007/s00412-005-0028-2
Rieder CL, Alexander SP (1990) Kinetochores are transported polewards along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J Cell Biol 110:81–85. doi:10.1083/jcb.110.1.81
Rieder CL, Salmon ED (1994) Motile kinetochores and polar ejection forces dictate chromosome position on the vertebrate mitotic spindle. J Cell Biol 124:223–233. doi:10.1083/jcb.124.3.223
Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM (2003) Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol 51:599–609. doi:10.1038/ncb0703-599
Rogers GC, Rogers SL, Schwimmer TA, Ems-McClung SC, Walczak CE, Vale RD, Scholey JM, Sharp DJ (2004) Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase. Nature 427:364–370. doi:10.1038/nature02256
Rogers GC, Rogers SL, Sharp DJ (2005) Spindle microtubules in flux. J Cell Sci 118:1105–1116. doi:10.1242/jcs.02284
Sampson K, Pickett-Heaps JD (2001) Phallicidin stains the kinetochore region in the mitotic spindle of the green algae Oedogonium sp. Protoplasma 217:166–176. doi:10.1007/BF01283397
Sampson K, Pickett-Heaps JD, Forer A (1996) Actin is involved in chromosomal attachment to the spindle and possibly anaphase A movement of chromosomes in the green alga Oedogonium. Protoplasma 192:130–144. doi:10.1007/BF01273885
Schibler MJ, Pickett-Heaps JD (1980) Mitosis in Oedogonium: spindle microfilaments and the origin of the kinetochore fiber. Eur J Cell Biol 22:687–698
Schrader F (1944) Mitosis. Columbia University Press, New York
Spurck T, Forer A, Pickett-Heaps JD (1997) Ultraviolet microbeam irradiations of epithelial and spermatocyte spindles suggest that forces act on the kinetochore fibre, and are not generated by its disassembly. Cell Motil Cytoskeleton 36:136–148. doi:10.1002/(SICI)1097-0169(1997)36:2<136::AID-CM4>3.0.CO;2-7
Tippit DH, Pickett-Heaps JD, Leslie R (1980) Cell division in two large pennate diatoms Hanzschia and Nitzschia. III. A new proposal for kinetochore function during prometaphase. J Cell Biol 86:402–416. doi:10.1083/jcb.86.2.402
Travis J (2007) The nucleus: the return of the matrix. Science 318:1400–1401. doi:10.1126/science.318.5855.1400
Uehara R, Hosoya H, Mabuchi I (2008) In vivo phosphorylation of regulatory light chain of myosin II in sea urchin eggs and its role in controlling myosin localization and function during cytokinesis. Cell Motil Cytoskeleton 65:100–115
Wadsworth P, Khodjakov A (2004) E pluribus unum: towards a universal mechanism for spindle assembly. Trends Cell Biol 14:413–419. doi:10.1016/j.tcb.2004.07.004
Wilson EB (1928) The cell in development and heredity. Macmillan, New York
Wittmann T, Waterman-Storer CM (2001) Cell motility: can Rho GTPases and microtubules point the way? J Cell Sci 114:3795–3803
Woolner S, O’Brien LL, Wiese C, Bement WM (2008) Myosin-10 and actin filaments are essential for mitotic spindle function. J Cell Biol 182:77–88. doi:10.1083/jcb.200804062
Zhang D, Nicklas RB (1996) ‘Anaphase’ and cytokinesis in the absence of chromosomes. Nature 382:466–468. doi:10.1038/382466a0
Acknowledgements
We greatly value the reviewing of this manuscript and suggestions by Dr. Jan Sapp. A.F. was supported by grants from the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
We dedicate this article to our dear friend and colleague Tim Spurck, who died suddenly on January 13, 2009, at age 53. We miss him.
Rights and permissions
About this article
Cite this article
Pickett-Heaps, J., Forer, A. Mitosis: spindle evolution and the matrix model. Protoplasma 235, 91–99 (2009). https://doi.org/10.1007/s00709-009-0030-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-009-0030-2