Summary.
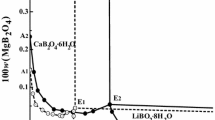
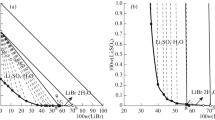
The isothermal solubility diagrams of four aqueous systems containing lithium nitrate and nitrates of group IIA metals – magnesium, calcium, strontium, and barium – were studied at 25°C. No double salt formation was observed. The results were compared with similar nitrate and chloride systems. Some trends in the shape of the phase diagrams were observed. Hydration analysis was applied to the solubility branches, rendering information about ionic processes in saturated solutions. Further, the ratio of activity coefficients of the saturating solid phase in ternary and binary solutions (γ/γ0) was obtained.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received April 13, 2001. Accepted June 15, 2001
Rights and permissions
About this article
Cite this article
Zbranek, V., Eysseltová, J. Study of the Ternary Systems M(II)(NO3)2–LiNO3–H2O (M(II) 5 Mg, Ca, Sr, Ba) at 25°C. Monatshefte für Chemie 132, 1463–1475 (2001). https://doi.org/10.1007/s007060170003
Issue Date:
DOI: https://doi.org/10.1007/s007060170003