Abstract
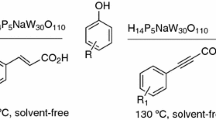
Tris(pentafluorophenyl)borane [B(C6F5)3] catalyzed simple, efficient and environmentally benign protocol has been developed for the Pechmann condensation using variety of phenols and β-ketoesters under solvent-free conditions to afford coumarin derivatives. The present protocol displayed significant advantages such as low catalyst loading, short reaction time, mild reaction conditions, low toxicity, easy work-up, high yields, and compatibility with other functional groups. In addition, it is a convenient, clean, and fast alternative approach for synthesizing variety of coumarin derivatives. Moreover, the applicability of this method towards large-scale synthesis demonstrated its suitability for the industrial application.
Graphic abstract

Similar content being viewed by others
References
Murray R (1995) Nat Prod Rep 12:477
Scheel LD (1972) Microbiol Toxins 8:47
Sun WC, Gee KR, Haugland RP (1998) Bioorg Med Chem Lett 8:3107
Wang CJ, Hsieh YJ, Chu CY, Lin YL, Tseng TH (2002) Cancer Lett 183:163
Bravic G, Gaultier J, Hauw C (1968) C R Acad Sci. Paris Ser IIc: Chim 267:1790
Arora RB, Mathur CN (1963) Br J Pharmacol Chemother 20:29
Palmer CJ, Josephs JL (1995) J Chem Soc Perkin Trans 1:3135
Maddi V, Kallur RS, Rao MNA (1992) J Pharm Sci 81:964
Musiciki B, Periers AM, Laurin P, Ferroud D, Benedetti Y, Lachaud S, Chatreaux F Haesslein JL, LLtis A, Pierre C, Khider J, Tessol N, Airault M Demassey J, Dupuis-Hamelin C Lassaigne P, Bonnefoy A, Vicat P, Klich M (2000) Bioorg Med Chem Lett 10:1695
Pechmann HV, Duisberg C (1884) Ber Dtsch Chem Ges 17:929
Johnson JR (1942) Org React 1:210
Shirner RL (1942) Org React 1:1
Brufola G, Fringuelli F, Piermatti O, Pizzo F (1996) Heterocycles 43:1257
Yavari I, Hekmat-Shoar, Zonouzi A (1998) Tetrahedron Lett 39:2391
Molnar M, Lončarić M, Kovač M (2020) Curr Org Chem 24:4
Appel H (1935) J Chem Soc 1031
Woods LL, Sapp J (1962) J Org Chem 27:3703
Ahmad ZS, Desai RD (1937) Proc Indian Acad Sci Chem Sci 5:277
Robinson R, Weygand F (1941) J Chem Soc 386
Nadkarni AJ, Kudav NA (1981) Ind J Chem Sect B 20:719
Valizadeha H, Shockravi A (2005) Tetrahedron Lett 46:3501
Bahekar SS, Shinde DB (2004) Tetrahedron Lett 45:7999
Karami B, Kiani M, Hoseini MA (2014) Chin J Catal 35:1206
Peng MM, Hemalatha P, Ganesh M, Palanichamy M, Jang HT (2014) J Ind Eng Chem 20:953
Sudha S, Venkatachalam K, Vishnu Priya S, Herbert Mabel J, Palanichamy M, Murugesan V (2008) J Mol Catal A: Chem 291:22
Heravi MM, Khorasani M, Derikvand F, Oskooie HA, Bamoharram FF (2007) Catal Commun 8:1886
Torviso R, Mansilla D, Belizan A, Alesso E, Moltrasio G, Vazquez P, Pizzio L, Blanco M, Caceres C (2008) Appl Catal A 339:53
Shirini F, Marjani K, Nahzomi HT (2007) Chin Chem Lett 18:909
Rezaei R, Dorosty L, Rajabzadeh M, Khalifeh R (2011) Chin Chem Lett 22:1313
Karami B, Kiani M (2011) Catal Commun 14:62
Nazeruddin GM, Pandharpatte MS, Mulani KB (2012) C R Chim 15:91
Albadi J, Shirini F, Abasi J, Armand N, Motaharizadeh T (2013) C R Chim 16:407
Li S, Qi X, Huang B (2016) Catal Today 276:139
Li TS, Zhang ZH, Yang F, Fu CG (1998) J Chem Res (S) 38
Abbasi Z, Rezayati S, Bagheri M, Hajinasiri R (2017) Chin Chem Lett 28:75
Sharma RK, Monga Y, Puri A (2013) Catal Commun 35:110
Zareyee D, Serehneh M (2014) J Mol Catal A: Chem 391:88
Yadav GD, Ajgaonkar NP, Varma A (2012) J Catal 292:99
Ahmed AI, El-Hakam SA, Khder AS, Abo El-Yazeed WS (2013) J Mol Catal A: Chem 366:99
Reddy BM, Patil MK, Lakshmanan P (2006) J Mol Catal A: Chem 256:290
Naik MA, Mishra BG, Dubey A (2008) Colloids Surf A 317:234
Ghodke S, Chudasama U (2013) Appl Catal A 453:219
Jadhav NH, Sakate SS, Rasal NK, Shinde DR, Pawar RA (2019) ACS Omega 4:8522
Potdar MK, Mohile SS, Salunkhe MM (2001) Tetrahedron Lett 42:9285
Frere S, Thiery V, Besson T (2001) Tetrahedron Lett 42:2791
Romanelli GP, Bennardi D, Ruiz DM, Baronetti G, Thomas HJ, Autino JC (2004) Tetrahedron Lett 45:8935
Laufer MC, Hausmann H, Holderich WF (2003) J Catal 218:315
Khaligh NG (2012) Catal Sci Technol 2:1633
Maheswara M, Siddaiah V, Damu GLV, Rao YK, Rao CV (2006) J Mol Catal A: Chem 255:49
Russell A, Frye JR (1941) Org Synth 21:22
Simmonis H, Remmert P (1914) Chem Ber 47:2229
Chandrasekhar S, Reddy CR, Chandrashekar G (2004) Tetrahedron Lett 45:6481
Chandrasekhar S, Reddy CR, Babu BN, Chandrashekar G (2002) Tetrahedron Lett 43:3801
Thirupathi P, Neupane LN, Lee KH (2011) Tetrahedron 67:7301
Chandrasekhar S, Chandrashekar G, Vijeender K, Reddy MS (2006) Tetrahedron Lett 47:3475
Chandrasekhar S, Reddy CR, Babu BN (2002) J Org Chem 67:9080
Prajapti SK, Nagarsenkar A, Babu BN (2014) Tetrahedron Lett 55:3507
Prajapti SK, Nagarsenkar A, Babu BN (2014) Tetrahedron Lett 55:1784
Guggilapu SD, Prajapti SK, Babu BN (2015) Tetrahedron Lett 56:889
Nagarsenkar A, Prajapti SK, Babu BN (2015) J Chem Sci 127:711
Prajapti SK, Gupta KK, Babu BN (2015) J Chem Sci 127:1047
Acknowledgements
The authors thank to NIPER, Hyderabad & Inovine Pharma Research Solution, Raipur for the scientific and instrumental support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prajapti, S.K., Rao, S.P. B(C6F5)3-catalyzed synthesis of coumarins via Pechmann condensation under solvent-free conditions. Monatsh Chem 152, 469–473 (2021). https://doi.org/10.1007/s00706-021-02747-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-021-02747-1