Abstract
This paper presents a novel, high sensitive analytical method for electrochemical determination of viloxazine at boron-doped diamond electrode using differential pulse voltammetry (DPV). The verification of viloxazine electrochemical behavior proved that viloxazine undergoes diffusion controlled, one-electron irreversible oxidation process. Optimization stage allowed for selection of acetate buffer solution (pH 5; 0.1 mol dm−3) as the best suitable supporting electrolyte and determination of DPV parameters. The calibration curve was obtained in the concentration range 0.1–20.0 µmol dm−3 with the detection limit equal to 0.04 µmol dm−3, respectively. The optimized procedure was validated by studying the sensitivity, accuracy, and precision of obtained results. The proposed method was successfully employed for viloxazine determination in spiked tap and river water samples with recovery of 95.8–98.8%.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, a significant increase in the consumption of various pharmaceuticals, including antidepressants, can be noticed. Depression is classified as chronic or recurrent mood disorder that affects about 121 million people all around the world [1]. The continuous increase in the consumption of antidepressants has been recorded in most of the well-developed countries. The Eurobarometer report from 2010 indicates that almost 7.5% of the European population use antidepressants regularly [2]. Such a large intake of antidepressants is also associated with increasing concentration of these drugs and their metabolites in the environment. Many of them are not effectively removed by wastewater treatment, thus they are getting into sources of drinking water, groundwater or bottom sediments [3,4,5,6,7]. World Health Organization in report from 2012 suggests that concentration of pharmaceuticals in environmental waters usually did not exceed 0.1 µg dm−3 [8]. However, numerous research conducted in different countries such as USA, Canada, China, or Denmark indicate the occurrence of much higher concentrations of antidepressants in surface waters, reaching even 10 µg dm−3, what is equivalent to approximately 50 nmol dm−3 [5, 9,10,11,12]. These observations prove that monitoring the presence of pharmaceutical contaminants in the environmental waters is extremely important. Thus, it is essential to develop analytical methods that allow the determination of antidepressants in both biological and environmental samples with high precision and accuracy.
2-[(2-Ethoxyphenoxy)methyl]morpholine, commonly known as viloxazine (Fig. 1), is a selective norepinephrine reuptake inhibitor (NRI) with minimal inhibitory effect on the reuptake of serotonin (5-HT) receptors. This drug has been widely used in the treatment of various types of depression, especially in the elderly with a tendency to delirium owing to minimal sedative anticholinergic and adrenergic effects. Viloxazine is almost completely absorbed following oral administration and in its unchanged form is carried by blood. Viloxazine is mainly excreted in the urine with about 12–15% being eliminated in unmetabolized form [13]. Considering the relatively poor degree of drug metabolism, it can be assumed that viloxazine could be found in the environmental waters.
Quantitative determination of viloxazine has been performed mainly in tablets and biological fluids, such as blood and urine [14,15,16]. So far, no one proposed a procedure to determine trace amounts of this drug in environmental water samples. Previously published methods for viloxazine determination include gas chromatography with flame ionization [15], electron capture [17], or nitrogen–phosphorus [18, 19] detection and high-performance liquid chromatography with fluorescence [14] and UV spectrophotometric [16] detection. Only one work proposed analytical method for viloxazine determination based on voltammetric techniques, precisely square-wave voltammetry measurements performed at glassy carbon electrode [20].
In recent years carbon-based electrodes have gained in importance and definitely outweigh the noble metal electrodes for electrochemical characteristics of organic and biological molecules. The main benefits of such materials are their electrocatalytic activity for a variety of redox reactions, low cost, wide potential window, and the possibility of modification with porous materials with large surface areas. Graphite and glassy carbon electrodes are, however, also known to suffer from surface fouling and oxide formation by reaction with oxygen and water. These surface reactions can have a significant effect on adsorption and electron-transfer kinetics [21].
Boron-doped diamond (BDD) electrodes have become attractive materials for electrochemical applications instead of glassy carbon or graphite due to their wider potential window, low background currents, high chemical inertness, high thermal conductivity, and high mechanical stability. Their superior overpotential for both oxygen and hydrogen is responsible for the widest potential window measured in aqueous electrolytes so far. Thus it gives the opportunity to study compounds with high oxidation and reduction potentials [22]. Owing to these properties, boron-doped diamond electrodes are well-known for their low adsorption, resistance to (bio)fouling, excellent response stability, and low background, which made them popular for a wide range of electroanalytical applications for both inorganic and organic compounds [23,24,25,26,27].
The aim of this work was to develop new analytical procedure for viloxazine determination and investigate their electrochemical behavior at boron-doped diamond electrode performing cyclic voltammetry (CV) and differential pulse voltammetry (DPV) measurements. The usefulness of obtained method was examined by determination of viloxazine in spiked environmental water samples.
Results and discussion
Electrochemical behavior of viloxazine
The electrochemical behavior of viloxazine was verified using cyclic and differential pulse voltammetry measurements performed using BDD electrode as a working electrode. As can be seen from cyclic voltammograms (Fig. 2a), viloxazine undergoes an irreversible oxidation process with one anodic wave observed at the potential close to 1.2 V. The application of a more sensitive DPV technique allowed to observe the formation of one anodic wave, recorded at 1.23 V and additionally, in some cases, peak shoulder at potential of ca. 1.07 V (Fig. 2b). The poorly shaped small wave visible at the base of the viloxazine oxidation peak has not been explained and interpreted in this work. It was observed only in DPV measurements performed without accumulation step or for viloxazine concentration exceeded 10 µmol dm−3. The appearance of the peak shoulder is probably related to rapid adsorption processes of the substrate and the reaction product on the surface of the working electrode resulting from the impulsive character of potential changes, characteristic for DPV technique.
Cyclic (a) and differential pulse (b) voltammograms registered for phosphate buffer solution (pH 7; 0.1 mol dm−3) (dotted line) and containing 20 µmol dm−3 viloxazine solution (solid line); CV parameters: scan rate 0.1 V s−1; DPV parameters: potential step 8 mV, pulse width 40 ms (20 ms of waiting time and 20 ms for current sampling time), pulse amplitude 50 mV, without accumulation step
The influence of the supporting electrolyte on the viloxazine oxidation peak current was also investigated. For this purpose cyclic voltammograms were recorded in a broad pH range (4–10) in 10 µmol dm−3 viloxazine solution, using as a supporting electrolyte three types of buffer solutions—acetate buffer (pH 4 and 5), phosphate buffer (pH 5, 6, 7, 8), and ammonium buffer (pH 8, 9, 10). Recorded voltammograms were subjected to the baseline correction and based on them, dependence of peak current and peak potential (Ep) in a function of pH value and concentration of buffer solution were determined (Fig. 3). Obtained results proved that the oxidation peak current is highly dependent on pH value of the solution used as a supporting electrolyte. It can be observed that peak current evenly grows with decreasing pH, and reaches maximum value for pH equal to 5 (Fig. 3a). Simultaneously, with declining pH the peak potential shifted towards more positive values (Fig. 3b), which proved the participation of protons in occurring electrode reaction. The dependence between peak potential and pH was linear with the slope of − 59.53 mV/pH, indicating the involvement in the viloxazine oxidation process the equal number of electrons and protons [28]. Moreover, type of used buffer solution influenced the registered current—application of acetate buffer instead of phosphate buffer with the same pH value allowed to obtain the highest peak current. Concentration of acetate buffer solution affected the recorded oxidation peak current as well (Fig. 3c). It can be noted that height of peak current decrease with the lowering of buffer concentration. Accordingly, acetate buffer at pH 5 and concentration 0.1 mol dm−3 was chosen as the best supporting electrolyte and was used in further studies.
Influence of pH value on registered viloxazine oxidation peak current (a) and peak potential (b) in CV measurements (scan rate 0.1 V s−1) obtained in the presence of 10 µmol dm−3 viloxazine in acetate buffer solution (pH 5; 0.1 mol dm−3); c recorded cyclic voltammograms (scan rate 0.1 V s−1; curves subjected to baseline correction) in acetate buffer (pH 5) with a concentration of 0.1 (I), 0.05 (II), 0.01 (III), and 0.005 (IV) mol dm−3 in the presence of viloxazine (10 µmol dm−3)
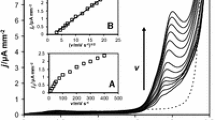
According to Randles–Sevčik equation, peak current (Ip) depends on square root of the scan rate (ν), therefore, this relationship should be linear when oxidation process is controlled by the diffusion [29]. The verification of scan rate impact on the recorded oxidation peak current and peak potential was performed in the range from 0.0125 to 0.5 V s−1 (Fig. 4a). The dependence of the peak current on square root of the scan rate (Fig. 4b) assumed the linear correlation, which is consistent with Randles–Sevčik equation, then the viloxazine oxidation can be considered as a diffusion-controlled process.
To verify the path of occurring electrode reaction, based on the recorded voltammograms, the influence of scan rate on the oxidation peak potential (Ep) was examined (Fig. 4c). It was observed that oxidation peak shifted towards more positive potentials with increasing scan rate, what is expected for an irreversible oxidation process [29]. The dependence of Ep in function of ln(ν) assumed the linear dependence with the slope equal to 0.0281. For an irreversible, diffusion-controlled process the relationship between oxidation peak potential and natural logarithm of scan rate can be described by equation [30]:
where Ep—peak potential [V], A—constant value, R—gas constant (R = 8.314 J mol−1 K−1), T—temperature [K], α—charge transfer coefficient, n—number of electrons, F—Faraday constant F = 96,485 C mol−1, ν—scan rate [mV s−1].
Based on the obtained slope value for dependence presented in Fig. 3c, the number of the electron transfer was calculated. Assuming standard value of charge transfer coefficient for irreversible process equal to 0.5, number of electrons was calculated as 0.914, which confirmed one-electron transfer for viloxazine oxidation process.
The correctness of determined number of electrons participating in the electrode reaction was verified by calculating n on the base of second, independent equation [30]:
where Ep/2 is a potential where the current is at half the peak value (other symbols explained with Eq. 1). The calculated potential difference (Ep− Ep/2) for analyzed reaction was equal to 85.6 mV (for viloxazine oxidation peak recorded at scan rate 0.1 mV s−1), which gives the electrons number value equal to 1.114, thus confirming the participation of one electron in viloxazine oxidation process.
Previously suggested mechanism of viloxazine oxidation indicates that the recorded anodic wave proceeds most likely via formation of cation-radical intermediate in aromatic part of molecule through a one-electron transfer occurring without protons participation [20]. Results and calculations obtained in this work suggest that electrode reaction proceeds rather via oxidation of secondary amine group through a one-electron transfer, accompanied by the release of one proton. These conclusions are consistent with previous literature reports related to secondary amine derivatives oxidation [31,32,33]. The mechanism of proposed electrode reaction is presented as Fig. 5.
It can be also noted that cyclic voltammetry measurements indicate the electrode reaction as diffusion-controlled process. However, DPV measurements suggest that viloxazine oxidation is preceded by adsorption of its molecules on the electrode surface. These observations leads to the conclusion that viloxazine oxidation performed during DPV measurements could be considered as both adsorption- and diffusion-controlled processes.
Optimization of experimental parameters
For quantitative determination of viloxazine the differential pulse voltammetry was selected as the suitable analytical technique. Therefore, DPV parameters, such as potential step (Es), pulse width (ts), and pulse amplitude (dE), were optimized without accumulation step.
To choose the best potential step value, DP voltammograms for different values of potential step were recorded, applying pulse width equal to 40 ms and pulse amplitude of 50 mV with previously developed measurement conditions (Fig. 6a). For each value of the changing parameter measurements were repeated three times and standard deviation of recorded peak current was calculated. It can be observed that with the growth of the potential step, the peak current also increases, which is related to the faster change of potential, so the larger scan rate. The highest and the most repeatable peak current value was obtained for potential step equal to 7 mV, therefore, this value was chosen for further studies.
Influence of DPV parameters: potential step (a), pulse width (b), and pulse amplitude (c) on viloxazine oxidation peak current recorded in acetate buffer solution (pH 5; 0.1 mol dm−3) containing 10 µmol dm−3 of viloxazine; inserts: DP voltammograms (subjected to baseline correction) achieved for different values of each optimized parameter. Optimization without accumulation step (tacc = 0 s)
For pulse width optimization, six values of pulse width (10, 20, 30, 40, 50, and 60 ms) were tested using previously chosen value of potential step and pulse amplitude of 50 mV. It can be noted (see Fig. 6b) that increasing pulse width extends the duration of each measurement, so reduces the scan rate. Therefore, with longer pulse width, lower peak current values were obtained. Taking into account the height of peak current and repeatability of measurements the best results were achieved for pulse width equal to 20 ms, which was equivalent to 10 ms of waiting time and 10 ms of current sampling time. Consequently, this value of pulse width was applied in following measurements.
Pulse amplitude value was chosen on the basis of DP voltammograms registration using pulse amplitude values between 10 and 80 mV (both positive and negative modes), with previously chosen potential step and pulse width, 7 mV and 20 ms, respectively (Fig. 6c). It can be seen that peak current grown with the higher absolute values of pulse amplitude. However, with a growth of pulse amplitude repeatability of measurements decreases. In case of negative modes of pulse amplitude, lower peak current with worse repeatability was obtained than for corresponding positive modes. Eventually, 50 mV was chosen as an optimal value of this parameter.
Additionally, other voltammetric parameters such as potential (Eacc) and time (tacc) of preconcentration were taken into account to enhance recorded peak current. Six values of accumulation potential between 0.2 and 0.8 V were tested applying accumulation time equal to 5 s and previously chosen measurement conditions (Fig. 7a). The highest peak current was achieved for accumulation potential equal to 0.5 V. The improvement in peak current height was also achieved with growing accumulation time. In Fig. 7b it can be observed that for preconcentration time above 10 s subsequent growth of accumulation time did not affect the registered peak height. It was also noticed that for preconcentration time longer than 10 s the measurements repeatability significantly decreased, therefore, this value was chosen as an optimal value.
Dependence of accumulation potential for tacc = 5 s (a), and accumulation time for Eacc = 0.5 V (b) on oxidation peak current registered using DPV technique in acetate buffer solution (pH 5; 0.1 mol dm−3) in the presence of 10 µmol dm−3 viloxazine (potential step 7 mV; pulse width 20 ms; pulse amplitude 50 mV); insert: DPV curves (subjected to baseline correction) registered for different accumulation time values
Analytical characteristics of developed method
The quantitative measurements, under previously selected experimental parameters, were performed for viloxazine in concentration range from 0.1 to 20.0 µmol dm−3 (Fig. 8). Obtained analytical data were summarized in Table 1. The limit of detection (LOD) was evaluated as a 3.3 times standard deviation of results obtained for the 0.1 µmol dm−3 viloxazine concentration (n = 5) over the calibration slope [34]. Then the quantification limit (LOQ) was calculated as 3 LOD. The calculated values of LOD and LOQ are also presented in Table 1.
Calibration graph achieved from DPV measurements for different concentrations of viloxazine in acetate buffer solution (pH 5; 0.1 mol dm−3), insert: DP voltammograms (subjected to baseline correction) corresponding to each concentration (potential step 7 mV; pulse width 20 ms; pulse amplitude 50 mV; tacc= 10 s; Eacc = 500 mV)
Limit of detection and linear range were compared with other previously reported methods for viloxazine determination (Table 2). The analytical procedure obtained in this work offers high sensitivity and relatively wide linear range. The obtained limit of detection is much lower in comparison to results described in literature so far, even for chromatographic methods. Despite the fact that indicated by WHO concentration levels of pharmaceuticals in environment are lower than achieved LOD, in relation to reported concentrations of various antidepressants in waters [5, 9,10,11,12], developed method gives possibility for detection and quantification of viloxazine in environmental water samples.
Interference studies
The selectivity of developed method was tested by examining the influence of different organic and inorganic compounds, which could be present in environmental water samples, on the viloxazine oxidation peak current. As possible interferents Triton X-100 (non-ionic surfactant), sodium dodecyl sulfate (SDS; anionic surfactant), cetrimonium bromide (CTAB; cationic surfactant), humic acid and inorganic ions (Na+, K+, Mg2+, Cl−, NO3−, SO42−) were chosen. To implement this part of the research, appropriate volumes of 0.1% standard solution of each organic compound were added to supporting electrolyte containing 10 µmol dm−3 of viloxazine, obtaining concentrations of interferents equal to 0.1, 1.0, 10, 20, and 50 µmol dm−3. For inorganic salts, 0.1 mol dm−3 stock solutions were used analogically as described above, resulting in 10-, 50-, 100-, and 500-fold excess of possible interferent in comparison to viloxazine concentration. For each tested substance a percentage difference between peak current registered for viloxazine without interferent and with the presence of interferent was calculated. The limit of tolerance was specified as percentage difference less than ± 5%.
It was observed that presence of any tested inorganic ion in supporting electrolyte solution did not affect the recorded viloxazine oxidation peak current. Even in the case of 500-fold excess of inorganic interferents in comparison to viloxazine concentration, obtained peak current differed less than 1%, which does not exceed the limit of the acceptable signal change. Also in the case of humic acid and anionic surfactant (SDS) no visible differences between height of the recorded peaks were observed within tested concentration range of these interferents. Only the presence of Triton X-100 and CTAB affected the registered viloxazine oxidation peak height (Fig. 9). It is clearly visible that increasing concentration of these surfactants obstructed the electrode reaction, and when their concentration exceeded the viloxazine concentration, recorded signal decreased by more than 20%. However, for lower concentrations of Triton X-100 and CTAB, no significant impact on the obtained oxidation peak current, with signal change less than 5%, was observed.
Interference studies. The signal changes caused by different concentrations of Triton X-100 (light gray) and cetrimonium bromide (dark gray) present in acetate buffer solution (pH 5; 0.1 mol dm−3) containing 10 µmol dm−3 viloxazine in DPV measurements (potential step 7 mV; pulse width 20 ms; pulse amplitude 50 mV; tacc = 10 s; Eacc= 500 mV)
Achieved results prove that the proposed method is characterized by good selectivity. The possible interference risk resulting from the presence of non-ionic and cationic surfactants in water samples can be compensated by employing of adequate calibration approach, e.g., integrated calibration [35] or generalized calibration strategy [36], which enables the specification of matrix effects and obtaining the accurate results of analysis.
Determination of viloxazine in water samples
For verification of proposed analytical method, the quantification of viloxazine in spiked tap and river water samples was carried out. Each tested water sample was spiked with viloxazine to obtain final concentration of 0.5, 1.0, and 2.0 µmol dm−3, respectively (see “Samples preparation”). The measurements were carried out in accordance with the procedure described in procedure of measurements. For each prepared spiked sample the viloxazine quantification procedure was repeated three times and then the mean value with confidence intervals (with significance level 0.05) was calculated. The results from viloxazine determination are presented in Table 3.
It was observed that shape, potential, and height of viloxazine oxidation peak recorded registered for spiked water samples did not differ from peaks recorded for the same viloxazine concentration at previous stages of conducted research. It suggests that matrix of tested water samples did not affect the signals received.
The recovery of viloxazine in water samples ranged from 95.8 to 98.8% with relative standard error (RSD) varying in the range from 2.2 to 6.1%. The confidence intervals obtained for recovery values do not exceed 5.0% what proves the acceptable repeatability. Good accuracy and precision confirm analytical usefulness of the developed method for viloxazine determination in environmental water samples.
Conclusion
A novel electroanalytical method for viloxazine determination using boron-doped diamond (BDD) electrode was developed. Application of BDD electrode, instead of commonly used glassy carbon electrodes, reduced background current, thus enhanced the sensitivity of method and allowed for simple, fast, and accurate determination of viloxazine in spiked water samples without any special sample pretreatment and any modification of working electrode surface. Presented work is second electrochemical method elaborated for viloxazine determination, distinguished by the lowest limit of detection obtained so far, even in comparison to chromatographic methods. The reproducibility is acceptable, with RSD values lower than 6.1%. Good recovery (95.8–98.8%) proves that developed method can be used successfully for viloxazine determination in water samples.
Experimental
Apparatus
The voltammetric measurements were conducted with the M161 electrochemical analyzer (mtm-anko, Poland). The EALab 2.1 software was used for data acquisition. The measurements were carried in three-electrode system in quartz vessel of 10 cm3 volume covered with a plastic cover with four holes that matched the size of each electrode and an additional hole for adding standard solution. Boron-doped diamond electrode (BDD, Windsor Scientific, Great Britain) was used as a working electrode, silver chloride electrode with double coat (Ag/AgCl, saturated KCl/2 mol dm−3 NaNO3; mtm-anko) played a role of a reference electrode and platinum wire placed in Teflon holder was used as an auxiliary electrode. Each buffer solution was prepared using pH-meter CP-511 with combined glass electrode EPS-1 (Elmetron, Poland). Ultrasonic bath Sonic-3 (Polsonic, Poland) and magnetic stirrer (Wigo, Poland) were also used.
Reagents and materials
All aqueous solutions were prepared with distilled water from the HLP 5 system (Hydrolab, Poland) and chemicals of analytical grade purity. The standard stock solution of 1.0 mmol dm−3 viloxazine (LGC, Great Britain) was obtained by dissolving of an appropriate amount of drug in 2 cm3 of water and then stored in fridge. The 0.1 mol dm−3 acetate buffers at pH 4 and 5 were prepared by mixing appropriate volumes of acetic acid (POCH, Poland) and 0.1 mol dm−3 sodium acetate solution (Merck, Germany). The phosphate buffers (pH 5, 6, 7, and 8) were prepared by mixing appropriate volumes of 0.1 mol dm−3 disodium hydrogen phosphate solution (POCH, Poland) and 0.1 mol dm−3 sodium dihydrogen phosphate solution (Chempur, Poland). The 0.1 mol dm−3 ammonium buffers at pH 8, 9, and 10 were prepared by mixing appropriate volumes of ammonia (Merck, Germany) and 0.1 mol dm−3 ammonium chloride (Merck, Germany). For interference studies potassium chloride (POCH, Poland), sodium nitrate (Merck, Germany), magnesium sulfate (Merck, Germany), Triton X-100 (Sigma-Aldrich, USA), humic acid (Merck, Germany), sodium dodecyl sulfate (Sigma-Aldrich, USA), and cetrimonium bromide (Sigma-Aldrich, USA) were used.
Procedure of measurements
Before each measurement series on a given day, the BDD electrode was activated by applying the potential of − 3.0 V for 15 min in 0.5 mol dm−3 sulfuric acid solution. Between subsequent measurements electrode was washed with distilled water.
For verification of electrochemical behavior of viloxazine on boron-doped diamond electrode cyclic voltammetry and differential pulse voltammetry were used. The cyclic voltammograms were recorded in the potential range from 0.5 to 1.4 V with scan rate equal to 0.1 V s−1. The preliminary DP voltammograms were registered in the potential range from 0.5 to 1.55 V with potential step 8 mV, pulse width 40 ms (20 ms of waiting time and 20 ms for current sampling time) and pulse amplitude 50 mV. Measurements using both techniques were conducted in 5 cm3 of phosphate buffer solution at pH 7 and concentration equals 0.1 mol dm−3, as a supporting electrolyte.
The final, quantitative DPV measurements were performed using acetate buffer solution at pH 5 and concentration of 0.1 mol dm−3 as a supporting electrolyte. Voltammograms were recorded in the anodic direction from 0.5 to 1.55 V with potential step 7 mV, pulse width 20 ms (10 ms of waiting time and 10 ms for current sampling time) and pulse amplitude 50 mV. Before each DPV measurement preconcentration potential of 0.5 V for 10 s was applied to the working electrode. At that time solution in quartz vessel was being stirred (ca. 500 rpm) using magnetic stirring bar. For viloxazine determination, the standard addition method was chosen as a calibration procedure.
Samples preparation
The sample of river water was taken from the Vistula river in Krakow, subjected to filtration and stored in the fridge before analysis. Tap water sample was taken from the water supply network in Krakow and analyzed without previous preparation. For DPV determination of viloxazine in real sample, the solutions were prepared by adding of 2.5 cm3 of spiked waters to the 2.5 cm3 of supporting electrolyte.
References
Rani S, Malik AK, Rekhi H, Kaur R, Kaur RR (2016) Crit Rev Anal Chem 46:424
Lewer D, O’Reilly C, Mojtabai R, Evans-Lacko S (2015) B J Psych 207:221
Barnes KK, Kolpin DW, Furlong ET, Zaugg SD, Meyer MT, Barber LB (2008) Sci Total Enivron 402:192
Focazio MJ, Kolpin DW, Barnes KK, Furlong ET, Meyer MT, Zaugg SD, Barber LB, Thurman ME (2008) Sci Total Enivron 402:201
Shengliu Y, Xiaoman J, Xinghui X, Zhang H, Zheng S (2013) Chemosphere 90:2520
Niemi LM, Stencel KM, Murphy MJ, Schultz MM (2013) Anal Chem 85:7279
Langford KH, Thomas KV (2009) Enivron Int 35:766
World Health Organization (2012) Pharmaceuticals in drinking water. World Health Organization, Geneva
Schultz MM, Fulong ET (2008) Anal Chem 80:1756
Metcalfe CD, Chu S, Judt C, Li H, Oakes KD, Servos MR, Andrews DM (2010) Environ Toxicol Chem 29:79
Styrishave B, Halling-Sørensen B, Ingerslev F (2011) Environ Toxicol Chem 30:254
Lajeunesse A, Smyth SA, Barclay K, Sauvé S, Gagnon C (2012) Water Res 46:5600
Finder M, Brogden RN, Speight TM, Avery GS (1977) Drugs 13:401
Gillilan R, Mason WD (1981) J Pharm Sci 70:1980
Norman TR, Burrows GD, Davies BM (1979) Brit J Clin Pharmacol 8:169
Thomare P, Kergueris MF, Bourin M, Thomas L, Larousse C (1990) J Chromatogr B Biomed Sci Appl 529:494
Groppi A, Papa P (1985) J Chromatogr B Biomed Sci Appl 337:142
Fazio A, Crisafulli P, Primerano G, D’Agostino AA, Oteri G, Francesco P (1984) Ther Drug Monit 6:484
Martínez MA, Sánchez de la Torre C, Almarza E (2002) J Anal Toxicol 26:296
Garrido EMPJ, Garrido JMPJ, Esteves M, Santos-Silva A, Marques MPM, Borges F (2008) Electroanalysis 20:1454
Luong JHT, Male KB, Glennon JD (2009) Analyst 134:1965
McCreery RL (2008) Chem Rev 108:2646
Compton RG, Foord JS, Marken F (2003) Electroanalysis 15:1349
Kraft A (2007) Int J Electrochem Sci 2:355
Brych M, Skrzypek S, Kaczmarska K, Burnat B, Leniart A, Gutowska N (2015) Electrochim Acta 169:117
Brycht M, Vajdle O, Sipa K, Robak J, Rudnicki K, Piechocka J, Tasić A, Skrzypek S, Guzsvány V (2018) Ionics 24:923
Kowalcze M, Jakubowska M (2019) Anal Chim Acta 1045:98
Wang J (2001) Analytical electrochemistry, 2nd edn. VCH, New York
Gosser DK (1993) Cyclic voltammetry: simulation and analysis of reaction mechanisms. VCH, New York
Bard A, Faulkner LR (2001) Electrochemical methods: fundamentals and applications. Wiley, New York
Masaui M, Sayo H, Tsuda Y (1968) J Chem Soc B 9:973
Portis LC, Bhat VV, Mann CK (1970) J Org Chem 35:2175
Adanier A, Chehimi MM, Gallado I, Pinson J, Vila N (2004) Langmuir 20:8243
Shrivastava A, Gupta VB (2011) Chron Young Sci 2:21
Kościelniak P, Akcin G, Herman M, Kozak J, Wieczorek M (2003) Ann Chim 903:1045
Kościelniak P, Wieczorek M, Kozak J, Herman M (2011) Anal Lett 44:411
Acknowledgements
M.M. has been partly supported by the EU Project POWR.03.02.00-00-I004/16.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Madej, M., Kochana, J. & Baś, B. Determination of viloxazine by differential pulse voltammetry with boron-doped diamond electrode. Monatsh Chem 150, 1655–1665 (2019). https://doi.org/10.1007/s00706-019-2380-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-019-2380-6