Abstract
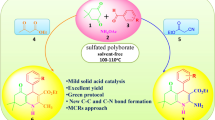
In this work, efficient, mild, and eco-friendly procedure of 1-aminoalkyl-2-phenol/Betti base from one-pot three-component condensations of aldehyde, phenol, and morpholine in the presence of sulfated polyborate catalyst, under a solvent-free condition at 100 °C, is described. The key advantages of the present method are short reaction time, high yields, aqueous work-up procedure, low cost, stable, reusable catalyst, and solvent-free reaction conditions.
Graphical abstract


Similar content being viewed by others
References
Betti M (1929) Org Synth 9:60
Betti M (1900) Gazz Chim Ital 30:310
Betti M (1901) Gazz Chim Ital 31:377
Betti M (1901) Gazz Chim Ital 31:170
Betti M (1901) Gazz Chim Ital 31:191
Betti M (1941) In: Gilman H (ed) Organic syntheses collective, vol I. John Wiley & Sons, New York, p 381
Betti M (1906) Gazz Chim Ital 36:392
Betti M (1906) Gazz Chim Ital 36:666
Gerlach M, Maul C (2007) Preparation of α-aminobenzylnaphthols and analogs as analgesics. U.S. Patent 7202242 B2, Apr 10, 2007; (2001) Chem Abstr 135:76698
Lu J, Xu X, Wang C, He J, Hu Y, Hu H (2002) Tetrahedron Lett 43:8367
Feng J, Bohle DS, Li CJ (2007) Tetrahedron Asymmetry 18:1043
Kidwai M, Chauhan R (2013) Asian J Org Chem 2:395
Gomez PG, Pabon HP, Carvajal MA, Rincon JM (1985) Rev Colomb Cienc Quim Farm 8:15
Waisser K, Gregor K, Kubicova L, Klimesova V, Kunes J, Machacek M, Kaustova (2000) Eur J Med Chem 35:733
Bouaziz Z, Riondel J, Mey A, Berlion M, Villard J, Filliond H (1991) Eur J Med Chem 26:469
Arthington SB, Motley AM, Warnock DW, Morrison CJ (2000) J Clin Microbiol 38:2254
Chylinska JB, Urbanski T, Mordarski M (1963) J Med Chem 6:484
Benameur L, Bouaziz Z, Nebois P, Bartoli MH, Boitard M, Fillion H (1996) Chem Pharm Bull 44:605
Mathew BP, Kumar A, Sharma S, Shukla PK, Nath M (2010) Eur J Med Chem 45:1502
Petrlíková E, Waisser K, Divišová H, Husáková P, Vrabcová P, Kuneš J, Kolář K, Stolaříková J (2010) Bioorg Med Chem 18:8178
Mojtahedi M, Shari A, Mohsenzadeh F, Saidi MR (2000) Synth Commun 30:69
Littman JB, Brode WR (1930) J Am Chem Soc 52:1655
Brode WR, Littman JB (1931) J Am Chem Soc 53:1531
Grumbach HJ, Arend M, Risch N (1996) Synthesis 1996:883
Katritzky AR, Abdel-Fattah Ashraf AA, Tymoshenko DO, Belyakov SA, Ghiviriga I, Steel PJ (1999) J Org Chem 64:6071
Safari J, Zarnegar Z (2013) J Mol Catal A Chem 379:269
Hajjami M, Ghorbani F, Bakhti F (2014) Appl Catal A 470:303
Hajipour AR, Ghayeb Y, Sheikhan N, Ruoho AE (2009) Tetrahedron Lett 50:5649
Zali A, Shokrolahi A (2012) Chin Chem Lett 23:269
Das B, Laxminarayana K, Ravikamth B, Rao BR (2007) J Mol Catal A Chem 261:180
Hashemi H, Sardarian AR (2013) J Iran Chem Soc 10:745
Wang M, Liang Y, Zhang TT, Gao JJ (2012) Chin Chem Lett 23:65
Khatri CK, Satalkar VB, Chaturbhuj GU (2017) Tetrahedron Lett 58:694
Khatri CK, Mali AS, Chaturbhuj GU (2017) Monatsh Chem 148:1463
Indalkar KS, Khatri CK, Chaturbhuj GU (2017) J Chem Sci 129:415
Khatri CK, Rekunge DS, Chaturbhuj GU (2016) New J Chem 40:10412
Indalkar KS, Khatri CK, Chaturbhuj GU (2017) J Chem Sci 129:141
Rekunge DS, Khatri CK, Chaturbhuj GU (2017) Tetrahedron Lett 58:1240
Indalkar KS, Khatri CK, Chaturbhuj GU (2017) Tetrahedron Lett 58:2144
Rekunge DS, Khatri CK, Chaturbhuj GU (2017) Monatsh Chem 148:2091
Patil MS, Palav AV, Khatri CK, Chaturbhuj GU (2017) Tetrahedron Lett 58:2859
Patil MS, Mudaliar C, Chaturbhuj GU (2017) Tetrahedron Lett 58:3256
Khatri CK, Chaturbhuj GU (2017) J Iran Chem Soc 14:2513
Khatri CK, Patil MS, Chaturbhuj GU (2017) J Iran Chem Soc 14:1683
Rekunge DS, Khatri CK, Chaturbhuj GU (2017) Tetrahedron Lett 58:4304
Indalkar KS, Patil MS, Chaturbhuj GU (2017) Tetrahedron Lett 58:4496
Jejurkar VP, Khatri CK, Chaturbhuj GU, Saha S (2017) ChemistrySelect 2:11693
Liu F, Li G, Jiang J, Zheng F, Wu M (2015) Tetrahedron Lett 56:5054
Karmakara B, Banerji J (2011) Tetrahedron Lett 52:4957
Kumar A, Gupta MK, Kumar M (2010) Tetrahedron Lett 51:1582
Rosholm T, Gois PMP, Franzen R, Candeias NR (2015) ChemistryOpen 4:39
Erb W, Albini M, Rouden J, Blanchet J (2014) J Org Chem 79:10568
Zhen M, Zhang D, Zhang Z, Peng Y (2016) ACS Comb Sci 18:697
Reddy BN, Rani CR, Reddy SM, Pathak M (2016) Res Chem Intermed 42:7533
Waghmode N, Kalbandhe AH, Thorat PB, Karade NN (2015) Tetrahedron Lett 12:117
Acknowledgements
The authors are grateful to the Department of Science and Technology, New Delhi, India for their financial supports.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, M.S., Khatri, C.K. & Chaturbhuj, G.U. Three-component, solvent-free synthesis of Betti base catalyzed by sulfated polyborate. Monatsh Chem 149, 1453–1457 (2018). https://doi.org/10.1007/s00706-018-2169-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2169-z