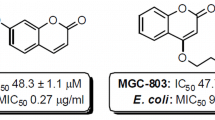
Abstract
A series of 5-[4-(4-aryl-1-piperazinyl)butoxy]coumarins were synthesized using microwave-assisted methods. The synthesized compounds were screened for their antibacterial activities against Gram-positive bacterial strains (Staphylococcus aureus, Micrococcus luteus, Bacillus cereus, Bacillus subtilis, Staphylococcus epidermidis, and Enterococcus hirae), Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa), and for their antifungal activities against three species of Candida (Candida albicans and Candida parapsilosis). The 4,7-dimethyl-5-[4-(pyridin-4-yl)butoxy]coumarin and the 6-acetyl-4,7-dimethyl-5-[4-(pyridin-4-yl)butoxy]coumarin were the most active against the Gram-positive bacteria. The best minimum inhibitory concentration values were obtained for the 4,7-dimethyl-5-[4-[4-[1-(4-pyridyl)]piperazin-1-yl]-butoxy]coumarin against Micrococcus luteus (15 µg/cm3). Two tested compounds exhibited moderate to good antifungal activity. The antitumor activity against of the newly synthesized compounds was evaluated. Among all the compounds tested, the 6-acetyl-4,7-dimethyl-5-[4-[4-(2-fluorophenyl)piperazin-1-yl]butoxy]coumarin was the most potent against HeLa cancer cells. Crystals of 4,7-dimethyl-5-[4-[4-(2-fluorophenyl)piperazin-1-yl]butoxy]coumarin were investigated using a single crystal X-ray diffraction technique.
Graphical abstract






Similar content being viewed by others
References
Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Nicolaides DN (2004) Curr Pharm Des 10:3813
Kashman Y, Gustafson KR, Fuller RW, Cardellina JH, McMahon JB, Currens MJ, Buckheit RW, Hughes SH, Cragg GM, Boyd MR (1993) J Med Chem 36:1110
Shikishima Y, Takaishi Y, Honda G, Ito M, Takfda Y, Kodzhimatov OK, Ashurmetov O, Lee KH (2001) Chem Pharm Bull 49:877
Gage BF (2006) Am Soc Hematol Educ Program: Hematology 467
Gormley NA, Orphanides G, Meyer A, Cullis PM, Maxwell A (1996) Biochemistry 35:5083
Ostrov DA, Prada JA, Corsino PE, Finton KA, Le N, Rowe TC (2007) Antimicrob Agents Chemother 51:3688
Riveiro ME, Moglioni A, Vazquez R, Gomez N, Facorro G, Piehl L, de Celis ER, Shayo C, Davio C (2008) Bioorg Med Chem 16:2665
Feuer G, Kellen JA, Kovacs K (1976) Oncology 33:35
Askin D, Eng KK, Rossen K, Purick RM, Wells KM, Volante RP, Reider PJ (1994) Tetrahedron Lett 35:673
Rossen K, Weissman SA, Sagar J, Reamer RA, Askin DA, Volante RP, Reider PJ (1995) Tetrahedron Lett 36:6419
Kimura M, Masuda T, Yamada K, Kobuta N, Kawakatsu N, Mitani M, Kishii K, Inazu M, Namiki T (2002) Bioorg Med Chem Lett 2:1947
Ryckebusch A, Poulain R, Maes L, Debreu-Fontaine MA, Mouray E, Grellier P, Sergheraert CJ (2003) Med Chem 46:542
Upadhayaya RS, Sinha N, Jain S, Kishore N, Chandra R, Arora SK (2004) Bioorg Med Chem 12:2225
Martin EG, Elgin RJ Jr, Mathiasen JR, Davis CB, Kesslick JM, Baldy WJ, Shank RP, Di Stefano DL, Fedde CL, Scott MKJ (1989) Med Chem 32:1052
Perrone R, Berardi F, Colabufo NA, Leopoldo M, Tortorella V (1999) J Med Chem 42:490
Glennon RA, Naiman NA, Lyon RA (1988) J Med Chem 31:1968
van Steen BJ, Wijngaarden I, Tulp M, Soudjin WJ (1993) Med Chem 36:2751
Glenon RA (1992) Drug Dev Res 26:247
Banno K, Fujioka T, Kikuchi T, Oshiro Y, Hiyama T, Nakagawa K (1988) Chem Pharm Bull 36:4377
Jaen JC, Wise LD, Heffner TG, Pugsley TA, Meltzer LTJ (1991) Med Chem 34:248
Santana L, Uriarte E, Fall Y, Teijeira M, Teran C, Garcıa-Martinez E, Tolf B (2002) Eur J Med Chem 37:503
Gonzalez-Gomez JC, Santana L, Uriarte E, Brea J, Villazon M, Loza MI, De Luca M, Rivas ME, Montenegro GY, Fontenla JA (2003) Bioorg Med Chem Lett 13:175
Rokosz LL, Huang CY, Reader JC, Stauffer TM, Chelsky D, Sigal NH, Ganguly AK, Baldwin JJ (2005) Bioorg Med Chem Lett 15:5537
Zeng S, Liu W, Nie FF, Zhao Q, Rong JJ, Wang J, Tao L, Qi Q, Lu N, Li ZY, Guo QL (2009) Biochem Biophys Res Commun 385:551
Mustafa MS, El-Abadelach MM, Zihilif MA, Naffa RG, Mubarak MS (2011) Molecules 16:4305
Mandala D, Valeru A, Pochampalli J, Vankadari SR, Tigulla P, Gatla R, Thampu R (2013) Med Chem Res 22:5481
Razavi SF, Khoobi M, Nadri H, Sakhteman A, Morad A, Emami S, Foroumadi A, Shafiee A (2013) Eur J Med Chem 64:252
Ostrowska K, Hejchman E, Wolska I, Kruszewska H, Maciejewska D (2013) Monatsh Chem 144:1679
Zawadowski T, Pfeffer J, Chęciński M (1980) Pol J Chem 54:1049
Hejchman E, Maciejewska D, Wolska I (2008) Monatsh Chem 139:1337
Pharm. Eur. 5 (2013) 2.7.2:188
Clinical and Laboratory Standards Institute (2012) 9th edn. CLSI publication M7-A9, Wayne, PA
Hejchman E, Ostrowska K, Kossakowski J, Courchesne WJ (2012) Pharm Exp Ther 342:380
Spackman MA, Jayatilaka D (2009) Cryst Eng Comm 11:19
Wolff SK, Grimwood DJ, McKinnon JJ, Turner MJ, Jayatilaka D, Spackman MA (2012) Crystal explorer (Version 3.1). University of Western Australia, Crawley
Spackman MA, McKinnon JJ (2002) Cryst Eng Comm 4:378
Bruker AXS Inc, Madison Wisconsin, USA
SAINT (2013) Bruker AXS Inc, Madison, Wisconsin, USA
SADABS (2012) Bruker AXS Inc, Madison, Wisconsin, USA
Sheldrick GM (1990) Acta Cryst A46:467
Sheldrick GM (2008) Acta Cryst A64:112
Wilson AJC (1992) International tables for crystallography, vol C. Kluwer, Dordrecht
(2009) Diamond 3.2—crystal and molecular structure visualization. In: Crystal impact, Bonn, Germany
Hejchman E, Taciak P, Kowalski S, Maciejewska D, Czajkowska A, Borowska J, Śladowski D, Młynarczuk-Biały (2015) I Pharmacol Rep 67:236
Thakar KA, Pathak RV, Dumir AB (1980) J Indian Chem Soc 57:89
Acknowledgments
This project was supported by Medical University of Warsaw, Faculty of Pharmacy, 2014/2015, FW24/NM1/14. The X-ray structure was determined in the Advanced Crystal Engineering Laboratory (aceLAB) at the Chemistry Department of the University of Warsaw.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ostrowska, K., Grzeszczuk, D., Maciejewska, D. et al. Synthesis and biological screening of a new series of 5-[4-(4-aryl-1-piperazinyl)butoxy]coumarins. Monatsh Chem 147, 1615–1627 (2016). https://doi.org/10.1007/s00706-016-1725-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1725-7