Abstract
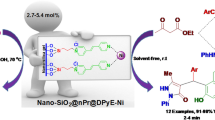
An efficient synthesis of 1,4-dihydropyridines was developed. 1,4-Dihydropyridines were synthesized starting from various 3-substituted isoxazolyl-5-carbaldehydes, ethyl acetoacetate, and ammonium acetate under microwave irradiation and solvent-free conditions (86–96 %), and were characterized by HRMS, FT-IR, 1H NMR, and 13C NMR spectroscopy. Solid support SiO2 was found to possess favorable catalysis and dispersancy for the condensation reaction. The merits of the method included the environmental friendly reaction conditions, simple operation, extensive substrates, good yields and reuse of the SiO2. Moreover, the crystal structure of compound diethyl 4-[3-(2-methoxyphenyl)isoxazol-5-yl]-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate in orthorhombic Pbca space group was presented.
Graphical abstract




Similar content being viewed by others
References
Vijesh AM, Isloor AM, Peethambar SK, Shivananda KN, Arulmolia T, Isloore NA (2011) Eur J Med Chem 46:5591
Natale NR, Triggle DJ, Palmer RB, Lefler BJ, Daniel Edwards W (1990) J Med Chem 33:2255
Wan JP, Lin YF, Jing YF, Xu ML, Liu YY (2014) Tetrahedron 70:7874
Koley S, Chowdhury S, Chanda T, Ramulu BJ, Samai S, Motisa L, Singh MS (2015) Tetrahedron 71:301
Barbachyn MR, Cleek GJ, Dolak LA, Garmon SA, Morris J (2003) J Med Chem 46:284
Pirrung MC, Tumey LN, Raetz CRH, Jackman JE, Snehalatha K (2002) J Med Chem 45:4359
Tanaka K, Inoue S, Murai N, Shirotori S, Nakamot K (2010) Chem Lett 39:1033
Kumar A, Maurya RA, Sharma S, Kumar M, Bhatia G (2010) Eur J Med Chem 45:501
Ulloora S, Shabaraya R, Ranganathan R, Adhikari AV (2013) Eur J Med Chem 70:341
Schade D, Lanier M, Willems E, Okolotowicz K, Bushway P, Wahlquist C, Gilley C, Mercola M, Cashman JR (2012) J Med Chem 55:9946
Locatelli A, Cosconati S, Micucci M, Leoni A, Marinelli L, Bedini A, Ioan P, Spampinato SM, Novellino E, Chiarini A, Budriesi R (2013) J Med Chem 56:3866
Niaz H, Kashtoh H, Khan JAJ, Khan A, Wahab A, Alam MT, Khan KM, Perveen S, Choudhary MI (2015) Eur J Med Chem 95:199
Budriesi R, Bisi A, Ioan P, Rampa A, Gobbi S, Belluti F, Piazzi L, Valenti P, Chiarini A (2005) Bioorg Med Chem 13:3423
Rucins M, Kaldre D, Pajuste K, Fernandes MAS, Vicente JAF, Klimaviciusa L, Jaschenko E, Kanepe-Lapsa I, Shestakova I, Plotniece M, Gosteva M, Sobolev A, Jansone B, Muceniece R, Klusa V, Plotniece A (2014) C R Chim 17:69
Bladen CM, Gündüz G, Şimşek R, Şafak C, Zamponi GW (2014) Pflugers Arch Eur J Physiol 466:1355
Refat HM, Fadda AA (2013) Eur J Med Chem 70:419
Hanaa AM (2013) Spectrochim Acta Part A 113:115
Fassihi A, Azadpour Z, Delbari N, Saghaie L, Memarian HR, Sabet R, Alborzi A, Miri R, Pourabbas B, Mardaneh J, Mousavi P, Moeinifard B, Sadeghi-aliabadi H (2009) Eur J Med Chem 44:3253
Abbas HAS, El Sayed WA, Fathy NM (2015) Eur J Med Chem 45:973
Singh RK, Prasad DN, Bhardwaj TR (2015) Med Chem Res 24:1534
Zhou X, Zhang L, Tseng E, Scott-Ramsay E, Schentag JJ, Coburn RA, Morris ME (2005) Drug Metab Dispos 33:321
Krauze A, Germane S, Eberlins O, Sturms I, Klusa V, Duburs G (1999) Eur J Med Chem 34:301
Donkor IO, Zhou XX, Schmidt J, Agrawal KC, Kishore V (1998) Bioorg Med Chem 6:563
Nikoorazm M (2013) Sci Iran C 20:603
Lavilla R (2002) J Chem Soc Perkin Trans 1:1141
Wan JP, Liu Y (2012) RSC Adv 2:9763
Bull JA, Mousseau JJ, Pelletier G, Charette AB (2012) Chem Rev 112:2642
Dam B, Nandi S, Pal AK (2014) Tetrahedron Lett 55:5236
Yoshida K, Inokuma T, Takasu K, Takemoto Y (2010) Synlett 12:1865
Moreau J, Duboc A, Hubert C, Hurvois JP, Renaud JL (2007) Tetrahedron Lett 48:8647
Maiti S, Sridharan V, Menendez JC (2010) J Comb Chem 12:713
Kantam ML, Ramani T, Chakrapani L, Choudary BM (2009) Catal Commun 10:370
Moghaddam FM, Mirjafary HSZ, Sadeghi A (2009) J Iran Chem Soc 6:317
Shi CL, Chen H, Shi DQ (2012) J Heterocycl Chem 49:125
Jiang HF, Mai RH, Cao H, Zhu QH, Liu XH (2009) Org Biomol Chem 7:4943
Heravi MRP, Aghamohammadi P (2012) C R Chim 15:448
Bartoli G, Babiuch K, Bosco M, Carlone A, Galzerano P, Melchiorre P, Sambri L (2007) Synlett 2897
Vohra RK, Bruneau C, Renaud JL (2006) Adv Synth Catal 348:2571
Wang LM, Sheng J, Zhang L, Han JW, Fan ZY, Tian H, Qian CT (2005) Tetrahedron 61:1539
Sridharan V, Perumal PT, Avendano C, Menéndez JC (2007) Tetrahedron 63:4407
Tewari N, Dwivedi N, Tripathi RP (2004) Tetrahedron Lett 45:9011
Wang XH, Hao WJ, Tu SJ, Zhang XH (2009) J Heterocycl Chem 46:742
Wen LR, Sun JH, Li M, Sun ET, Zhang SS (2008) J Org Chem 73:1852
Singh SK, Singh KN (2012) Monatsh Chem 143:805
Sathicq AG, Liberto NA, Fernandes SA, Romanelli GP (2015) C R Chim 18:374
Debache A, Ghalem W, Boulcina R, Belfaitah A, Rhouati S, Carboni B (2009) Tetrahedron Lett 50:5248
Tamaddon F, Razmi Z, Jafari AA (2010) Tetrahedron Lett 51:1187
Lee YA, Kim SC (2011) J Ind Eng Chem 17:401
Kuraitheerthakumaran A, Pazhamalai S, Gopalakrishnan M (2011) Chin Chem Lett 22:1199
Mirzaei H, Davoodnia A (2012) Chin J Catal 33:1502
Eynde JJV, Mayence A (2003) Molecules 8:381
Pasunooti KK, Jensen CN, Chai H, Leow ML, Zhang DW, Liu XW (2010) J Comb Chem 12:577
Zhang DW, Zhang YM, Zhang YL, Zhao TQ, Liu HW, Gan YM, Gu Q (2015) Chem Pap 69:470
Rajanarendar E, Ramesh P, Srinivas M, Ramu K, Mohan G (2006) Synth Commun 36:665
Li BZ, Gu Q, He YH, Zhao TQ, Wang SJ, Kang J, Zhang YM (2012) C R Chim 15:784
Liu JH, Yu LF, Eaton JB, Caldarone B, Cavino K, Ruiz C, Terry M, Fedolak A, Wang D, Ghavami A, Lowe DA, Brunner D, Lukas RJ, Kozikowski AP (2011) J Med Chem 54:7280
Natale NR, Rogers ME, Staples R, Triggle DJ, Rutledge A (1999) J Med Chem 42:3087
Mirzaei YR, Bavili-Tabrizi S, Hashemi-Gohare M, Zare-Neirizi H, Edjlali L (2003) Org Prep Proced Int 35:207
Bernstein J, Davis RE, Shimoni L, Chang NL (1995) Angew Chem Int Ed 34:1555
Sheldrick GM (1997) SHELXS-97, program for crystal structure solution. University of Göttingen, Göttingen
Sheldrick GM (1997) SHELXL-97, program for crystal structure refinement. University of Göttingen, Göttingen
Acknowledgments
We are grateful to Mr Ch. Y. Wang for NMR spectra, Mr Zh. L. Wei for mass spectra, and Ms L. Ye and Ms Q. Su for X-ray.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, D., Chen, X., Guo, X. et al. An efficient solvent-free synthesis of isoxazolyl-1,4-dihydropyridines on solid support SiO2 under microwave irradiation. Monatsh Chem 147, 1605–1614 (2016). https://doi.org/10.1007/s00706-016-1657-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1657-2