Abstract
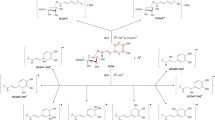
In this article, the antoxidative mechanisms HAT, SPLET, and SET-PT of the ortho-, meta-, and para-hydroxybenzoic acids and corresponding carboxylate anions with different radicals (·OO−, ·OH, ·OOH, and CH3OO·) were investigated. For this reason, the ΔH BDE, ΔH IP, and ΔH PA values of the corresponding reactions in different solvents (water, DMSO, pentylethanoate, and benzene) were examined. For this purpose, the M05-2X/6-311++G(d,p) and B3LYP-D2/6-311++G(d,p) theoretical models were applied. Although the B3LYP-D2 method produced lower reaction enthalpy values, both theoretical models exhibited the same trend. It was found that SET-PT is not a favorable reaction path for any hydroxybenzoic acids and their anions with any radicals in any solvents. No anion reacts with −·O2, whereas meta- and para-hydroxybenzoic acids react with −·O2 only in nonpolar solvents. In all other cases, the HAT and SPLET mechanisms are competitive. Which of them is dominant depends on the properties of the acids, anions, radicals, and solvents.
Graphical Abstract



Similar content being viewed by others
References
Amarowicz R, Fornal J, Karamać M (1995) Grasas Aceites 46:354
Naczk M, Amarowicz R, Sullivan A, Shahidi F (1998) Food Chem 62:489
Shahidi F, Wanasundara UN, Amarowicz R (1994) Food Res Int 27:489
Shahidi F, Naczk M (2004) Phenolics in food and nutraceuticals. CRC Press, Boca Raton
Weidner S, Amarowicz R, Karamać M, Dabrowski G (1999) Eur Food Res Technol 210:109
Weidner S, Amarowicz R, Karamać M, Fraczek E (2000) Plant Physiol Biochem 38:595
Sosulski F, Krygier K, Hogge L (1982) J Agric Food Chem 30:337
Herrmann K, Nagel CW (1989) Crit Rev Food Sci Nutr 28:315
Kim D-O, Lee CY (2004) Crit Rev Food Sci Nutr 44:253
Tanrioven D, Eksi A (2005) Food Chem 93:89
Sllbratte MA, Neergheen VS, Luximon-Ramma A, Aruona OI, Bahorum T (2005) Mutat Res 579:200
Matthews C, Davidson J, Bauer E, Morrison JL, Richardson AP (1956) J Am Pharm Assoc (Baltim) 45:260
Soni MG, Carabin IG, Burdock GA (2005) Food Chem Toxicol 43:985
Zhang J-D, Zhu Q-Z, Li S-J, Tao F-M (2009) Chem Phys Lett 475:15
Cuvelier M-E, Richard H, Berset C (1992) Biosci Biotech Biochem 56:324
Wright JS, Johnson ER, DiLabio GA (2001) J Am Chem Soc 123:1173
Klein E, Lukeš V, Ilčin M (2007) Chem Phys 336:51
Litwinienko G, Ingold KU (2007) Acc Chem Res 40:222
Di Meo F, Lemaur V, Cornil J, Lazzaroni R, Duroux J-L, Olivier Y, Trouillas P (2013) J Phys Chem A 117:2082
Košinova P, Di Meo F, El Anouar H, Duroux J-L, Trouillas P (2011) Int J Quantum Chem 111:1131
Yang YL, Dyakov Y, Lee YT, Ni C-K, Sun Y-L, Hu W-P (2011) J Chem Phys 134:034314
Yahagi T, Fujii A, Ebata T, Mikami N (2001) J Phys Chem A 105:10673
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick AD, Rabuck KD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (2009) Gaussian 09, revision A.1-SMP. Gaussian Inc, Wallingford
McLean AD, Chandler GS (1980) J Chem Phys 72:5639
Wachters AJH (1970) J Chem Phys 52:1033
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215
Zhao Y, Schultz NE, Truhlar DG (2005) J Chem Phys 123:161103
Alberto ME, Russo N, Grand A, Galano A (2013) Phys Chem Chem Phys 15:4642
Black G, Simmie JM (2010) J Comput Chem 31:1236
Galano A, Alvarez-Idaboy JR (2009) Org Lett 11:5114
Galano A, Macias-Ruvalcaba NA, Medina-Campos ON, Pedraza-Chaverri J (2010) J Phys Chem B 114:6625
Marković ZS, Dimitrić Marković JM, Doličanin ĆB (2010) Theor Chem Acc 127:69
Marković Z, Milenković D, Đorović J, Dimitrić Marković JM, Stepanić V, Lučić B, Amić D (2012) Food Chem 135:2070
Zavala-Oseguera C, Alvarez-Idaboy JR, Merino G, Galano A (2009) J Phys Chem A 113:13913
Marković Z, Milenković D, Đorović J, Dimitrić Marković JM, Stepanić V, Lučić B, Amić D (2012) Food Chem 134:1754
Grimme S (2011) WIREs Comput Mol Sci 1:211
Grimme S (2004) J Comput Chem 25:1463
Grimme S (2006) J Comput Chem 27:1787
Bayach I, Sancho-García JC, Di Meo F, Weber J-FF, Trouillas P (2013) Chem Phys Lett 578:120
Becke AD (1993) J Chem Phys 98:5648
Becke AD (1988) Phys Rev A 38:3098
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Marenich AV, Cramer CJ, Truhlar DG (2009) J Phys Chem B 113:6378
Carpenter JE, Weinhold F (1988) J Mol Struc (Theochem) 169:41
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899
Acknowledgments
The authors gratefully acknowledge financial support from the Ministry of Science of the Republic of Serbia (project Nos. 172015 and 174028) and the Ministry of Science, Education, and Sports of the Republic of Croatia (project Nos. 079-0000000-3211 and 098-0982464-2511).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marković, Z., Đorović, J., Dimitrić Marković, J.M. et al. Investigation of the radical scavenging potency of hydroxybenzoic acids and their carboxylate anions. Monatsh Chem 145, 953–962 (2014). https://doi.org/10.1007/s00706-014-1163-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-014-1163-3