Abstract
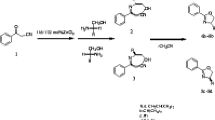
A new strategy which uses cheap K3PO4 as a very effective catalyst has been developed for synthesis of β-amino ketones by one-pot reaction of an aryl aldehyde, acetophenone derivative, and amine in EtOH at room temperature.
Graphical Abstract



Similar content being viewed by others
References
Abele S, Seebach D (2000) Eur J Org Chem 6:1
Hagiwara E, Fujii A, Sodeoka M (1998) J Am Chem Soc 120:2474
Seebach D, Matthews JL (1997) Chem Commun 2015
Drury WJ, Ferraris D, Cox C, Young B, Leckta T (1998) J Am Chem Soc 120:11006
Kleinmann EF (1991) Comprehensive Organic Synthesis. Pergamon, New York
Corey EJ, Reichard GA (1989) Tetrahedron Lett 30:5207
Reboule I, Gil R, Collin J (2005) Tetrahedron Lett 46:7761
Juaristi E (1997) Enantioselective synthesis of β-amino acids. Wiley-VCH, New York
Wang L, Han J, Sheng J, Tian H, Fan Z (2005) Catal Commun 6:201
Li H, Zeng HY, Shao HW (2009) Tetrahedron Lett 50:6858
Manabe K, Kobayashi S (1999) Org Lett 1:1965
Fang D, Gong K, Zhang DZ, Liu ZL (2009) Monatsh Chem 140:1325
Lu GP, Cai C (2010) Catal Commun 11:745
Kidwai M, Mishra NK, Bansal V, Kumar A, Mozumdar S (2009) Tetrahedron Lett 50:1355
Wang M, Song ZG, Jiang H (2009) Org Prep Proced Int 41:315
Azizi N, Saidi MR (2004) Tetrahedron 60:383
Varala R, Alam MM, Adapa SR (2003) Synlett 720
Jenner G (1995) Tetrahedron Lett 36:233
Srivastava N, Banik BK (2003) J Org Chem 68:2109
Bartoli G, Bosco M, Marcantoni E, Petrini M, Sambri L, Torregiani E (2001) J Org Chem 66:9052
Loh TP, Wei LL (1998) Synlett 975
Wabnitz TC, Spencer JB (2002) Tetrahedron Lett 43:3891
Chaudhuri MK, Hussain S, Kantam ML, Neeliam B (2005) Tetrahedron Lett 46:8329
Basu B, Das P, Hossain I (2004) Synlett 2630
Das B, Chowdhury N (2007) J Mol Catal A Chem 263:212
Hashemi MM, Eftekhari-Sis B, Abdollahifar A, Khalili B (2006) Tetrahedron 62:672
Surendra K, Krishnaveni NS, Sridhar R, Rama Rao K (2006) Tetrahedron Lett 47:2125
Varala R, Sreelatha N, Adapa SR (2006) Synlett 1549
Borah KJ, Phukan M, Borah R (2010) Synth Commun 40:2830
Firouzabadi H, Iranpoor N, Jafari AA (2005) Adv Synth Catal 347:655
Fetterly BM, Jana NK, Verkade JG (2005) Tetrahedron 61:1
Kantam ML, Neeraja V, Kavita B, Neelima B, Chaudhuri MK, Hussain S (2005) Adv Synth Catal 347:763
Ahmed N, van Lier JE (2006) Tetrahedron Lett 47:2725
Xu LW, Xia CG (2004) Synthesis 2191
Xu LW, Xia CG, Hu XX (2003) Chem Commun 2570
Yang L, Xu LW, Xia CG (2005) Tetrahedron Lett 46:3279
Ai X, Wang X, Liu JM, Ge ZM, Cheng TM, Li RT (2010) Tetrahedron 66:5373
Yang L, Xu LW, Zhou W, Li L, Xia CG (2006) Tetrahedron Lett 47:7723
Kantam ML, Neelima B, Reddy CV (2005) J Mol Catal A Chem 241:147
Movassagh B, Rakhshani A (2011) Chin Chem Lett 22:1179
Shirakawa E, Kibata T, Otsuka H, Tsuchimoto T (2005) Tetrahedron 61:9878
Beletskaya IP, Cheprakov AV (2000) Chem Rev 100:2009
Yao Q, Kinney EP, Yang Z (2003) J Org Chem 68:7528
Niu J, Zhou H, Li Z, Xu J, Hu S (2008) J Org Chem 73:7814
Niu J, Guo P, Kang J, Li Z, Xu J, Hu S (2009) J Org Chem 74:5075
Joshi AV, Bhusare S, Baidossi M, Qafisheh N, Sasson Y (2005) Tetrahedron Lett 46:3583
Yi WB, Cai C (2006) J Fluorine Chem 127:1515
Jafari AA, Moradgholi F, Tamaddon F (2009) Eur J Org Chem 1249
Bigdelli MA, Nemati F, Mahdavinia GH (2007) Tetrahedron Lett 48:6801
Wang R, Li BG, Huang TK, Shi S, Lu XX (2007) Tetrahedron Lett 48:2071
Zahouily M, Bahlaouan B, Rayadh A, Sebti S (2004) Tetrahedron Lett 45:4135
Mansour EME, El-Sadany SK, Kassem AA, Maksoud HA (1989) J Chem Eng Data 34:368
Zahouily M, Mounir B, Charki H, Mezdar A, Bahlaouan B, Ouammou M (2006) Arkivoc xiii:178
Li Z, Ma X, Liu J, Feng Z, Tian G, Zhu A (2007) J Mol Catal A Chem 272:132
Acknowledgments
We acknowledge the K.N. Toosi University of Technology Research Council for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Movassagh, B., Khosousi, S. K3PO4-catalyzed one-pot synthesis of β-amino ketones. Monatsh Chem 143, 1503–1506 (2012). https://doi.org/10.1007/s00706-012-0729-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-012-0729-1