Abstract
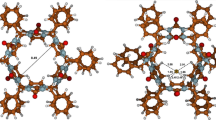
Quantum mechanical density functional theory (DFT) calculations were used to derive the most probable structures of the bambus[6]uril·H3O+ and bambus[6]uril·(H3O+)2 cationic complex species. In these two complexes, each of the considered H3O+ ions is bound by three strong linear hydrogen bonds to the three corresponding carbonyl oxygens of the parent macrocyclic receptor.
Graphical abstract





Similar content being viewed by others
References
Svec J, Necas M, Sindelar V (2010) Angew Chem Int Ed 49:2378
Toman P, Makrlík E, Vaňura P (2011) Monatsh Chem 142:881
Toman P, Makrlík E, Vaňura P (2011) Monatsh Chem 142:993
Makrlík E, Vaňura P (2006) Monatsh Chem 137:157
Makrlík E, Vaňura P (2006) Monatsh Chem 137:1185
Dybal J, Makrlík E, Vaňura P (2007) Monatsh Chem 138:541
Kříž J, Dybal J, Makrlík E, Budka J, Vaňura P (2007) Monatsh Chem 138:735
Dybal J, Makrlík E, Vaňura P, Selucký P (2007) Monatsh Chem 138:1239
Dybal J, Makrlík E, Vaňura P, Budka J (2008) Monatsh Chem 139:1175
Dybal J, Makrlík E, Budka J, Vaňura P (2008) Monatsh Chem 139:1353
Makrlík E, Dybal J, Vaňura P (2009) Monatsh Chem 140:29
Makrlík E, Dybal J, Budka J, Vaňura P (2009) Monatsh Chem 140:1155
Kříž J, Dybal J, Makrlík E, Budka J, Vaňura P (2010) Monatsh Chem 141:19
Makrlík E, Čajan M, Budka J, Vaňura P (2011) Monatsh Chem 142:5
Makrlík E, Vaňura P, Budka J (2009) Monatsh Chem 140:583
Toman P, Makrlík E, Vaňura P, Kašička V, Rathore R (2010) Monatsh Chem 141:737
Kříž J, Dybal J, Makrlík E (2006) Biopolymers 82:536
Kříž J, Dybal J, Makrlík E, Vaňura P, Lang J (2007) Supramol Chem 19:419
Kříž J, Dybal J, Makrlík E, Vaňura P (2008) Supramol Chem 20:387
Kříž J, Dybal J, Makrlík E, Budka J, Vaňura P (2008) Supramol Chem 20:487
Kříž J, Dybal J, Makrlík E, Budka J (2008) J Phys Chem A 112:10236
Kříž J, Dybal J, Makrlík E, Budka J, Vaňura P (2009) J Phys Chem A 113:5896
Kříž J, Toman P, Makrlík E, Budka J, Shukla R, Rathore R (2010) J Phys Chem A 114:5327
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Becke AD (1993) J Chem Phys 98:5648
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery Jr JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision C. 02, Gaussian, Wallingford CT
Acknowledgments
This work was supported by the Grant Agency of Faculty of Environmental Sciences, Czech University of Life Sciences, Prague, project No.: 42900/1312/3114 “Environmental Aspects of Sustainable Development of Society”, by the Czech Ministry of Education, Youth, and Sports (project MSM 6046137307), and by the Czech Science Foundation (project P 205/10/2280). The computer time at the MetaCentrum (project LM 2010005), as well as at the Institute of Physics (computer Luna/Apollo), Academy of Sciences of the Czech Republic, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toman, P., Makrlík, E. & Vaňura, P. Theoretical study on the protonation of bambus[6]uril. Monatsh Chem 143, 373–376 (2012). https://doi.org/10.1007/s00706-011-0682-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-011-0682-4