Abstract
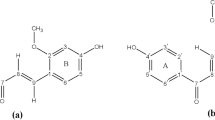
The structural properties of rutin were determined by using a computational multistep progression. In the first step a stochastic strategy based on a molecular mechanics calculation was adopted to obtain a preliminary screening of the low-energy rutin conformations. The most stable structures obtained by the random conformational search were used as a starting point for an Austin Model 1 (AM1) semiempirical optimization. After this treatment, 16 structures characterized by a more stable frontal in respect to back disposition of the glycosidic chain of rutin were identified. To validate the results found from the stochastic search a molecular dynamics simulation was carried out. The results evidenced the presence of a global minimum highly stabilized by a hydrogen bond between the hydroxyl in the 3′ position of the B ring and the endocyclic oxygen of the rhamnose unit followed by approximately 8 kJ mol−1 less stable local minima with similar energy values. Finally, the reliability of the molecular model was confirmed by comparing the calculated electronic absorption spectrum with that measured on a methanolic rutin solution.
Graphical abstract







Similar content being viewed by others
References
Harborne JB (1994) The Flavonoids: advances in research since 1986. Chapman and Hall, London
Harborne JB, Williams CA (2000) Phytochemistry 55:481
Dixon RA, Paiva NL (1995) Plant Cell 7:1085
Reuber S, Bornman JF, Weissenböck G (1996) Physiol Plant 97:160
Pfündel EE, Agati G, Cerovic ZG (2006) Optical properties of plant surfaces. In: Riederer M, Müller C (eds) Biology of the plant cuticle. Annu Plant Rev, vol 23. Blackwell, Oxford, p 215
Hernández I, Alegre L, Van Breusegem F, Munné-Bosch S (2009) Trends Plant Sci 14:125
Agati G, Matteini P, Goti A, Tattini M (2007) New Phytol 174:77
Ren W, Qiao Z, Wang H, Zhu L, Zhang L (2003) Med Res Rev 23:519
Wang HK (2000) Expert Opin Investig Drugs 9:2103
Rice-Evans CA, Miller NJ, Paganga G (1997) Trends Plant Sci 2:152
Cushnie TP, Lamb AJ (2005) Int J Antimicrob Agents 26:343
Havsteen BH (2002) Pharmacol Ther 96:67
Cody V, Middleton E, Harborne JB (1985) Plants flavonoids in biology and medicine: biochemical, pharmacological, and structure-activity relatioships. Alan R Liss, New York
Xi J, Guo R (2007) Int J Biol Macromol 40:305
Kanakis CD, Tarantilis PA, Polissiou MG, Diamantoglou S, Tajmir-Riahi HA (2006) J Mol Struct 798:69
Hodek P, Hanustiak P, Krízková J, Mikelova R, Krízková S, Stiborová M, Trnková L, Horna A, Beklová M, Kizek R (2006) Neuro Endocrinol Lett 27(Suppl 2):14
Papadopoulou A, Green RJ, Frazier RA (2005) J Agric Food Chem 2005:158
Zsila F, Bikádi Z, Simonyi M (2006) Biochem Pharmacol 65:447
Lin CM, Chen CS, Chen CT, Liang YC, Lin JK (2002) Biochem Biophys Res Commun 294:167
De Oliveira EB, Humeau C, Chebil L, Maia ER, Dehez F, Maigret B, Ghoul M, Engasser J-M (2009) J Mol Catal B Enzym 59:96
Cornard JP, Merlin JC, Boudet AC, Vrielynck L (1997) Biospec 3:183
Russo N, Toscano M, Uccella N (2000) J Agric Food Chem 48:3232
Amat A, De Angelis F, Sgamellotti A, Fantacci A (2008) J Mol Struct 868:12
Mielczarek C (2005) Eur J Pharm Sci 25:273
Cornard JP, Boudet AC, Merlin JC (1999) J Mol Struct 508:37
Allinger NL (1977) J Am Chem Soc 99:8127
Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM Jr, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA (1995) J Am Chem Soc 117:5179
Dewar MJS, Zoebisch EG, Healy EF, Stewart JJP (1985) J Am Chem Soc 107:3902
Dannenberg JJ, Evleth EM (1992) Int J Quant Chem 44:869
Karplus M (2003) Biopolymers 68:350
Sherrill CD, Schaefer HF III (1999) Adv Quant Chem 34:143
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matteini, P., Goti, A. & Agati, G. Theoretical conformational analysis of rutin. Monatsh Chem 141, 793–800 (2010). https://doi.org/10.1007/s00706-010-0330-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-010-0330-4