Abstract
Five novel lanthanide (Eu3+, Tb3+, Sm3+, Dy3+, and Gd3+) complexes with 5-nitro-1,10-phenanthroline (phenNO2) have been synthesized and characterized by elemental analysis, IR, UV, and luminescence spectra. The triplet state energy of phenNO2 was determined to be 20,048 cm−1 via the phosphorescence spectra of phenNO2 and its gadolinium complex. The photophysical properties of these complexes indicated that the triplet state energy of the ligand is suitable for the sensitization of the luminescence of Eu3+ and Sm3+, especially the former.
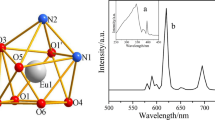
Graphical abstract
5-Nitro-1,10-phenanthroline acts as photon antenna able to transfer energy to Eu(III) ion effectively. Therefore, the intense typical fluorescence of the Eu(III) ions was observed







Similar content being viewed by others
References
Meares CF, Wensel TG (1984) Acc Chem Res 17:202
Scott LK, Horrocks WD Jr (1992) J Inorg Biochem 46:193
Yam VWW, Lo KKW (1999) Coord Chem Rev 184:157
Beeby A, Botchway SW, Clarkson IM, Faulkner S, Parker AW, Parker D, Williams JAG (2000) J Photochem Photobiol B Biol 57:83
Bazin H, Trinquet E, Mathis G (2002) Rev Mol Biotechnol 82:233
Xu CJ (2006) J Rare Earth 24:429
Jin LP, Lu SX, Lu SZ (1996) Polyhedron 15:4069
Zhang HJ, Yan B, Wang SB, Ni JZ (1997) J Photochem Photobiol A Chem 109:223
Xu CJ, Yang H, Xie F, Guo XZ (2005) J Alloys Compds 392:96
Brito HF, Malta OL, Felinto MCFC, Teotonio EES, Menezes JFS, Silva CFB, Tomiyam CS, Carvalho CAA (2002) J Alloys Compds 344:293
Corey EJ, Borror AL, Foglia T (1965) J Org Chem 30:288
Yamada M, Tanaka Y, Yoshimoto Y, Kuroda S, Shimao I (1992) Bull Chem Soc Jpn 65:1006
Hammett LP, Walden GH Jr, Edmonds SM (1934) J Am Chem Soc 56:1092
Chassapis C, Pneumatikakis G (1982) Inorg Chim Acta 59:49
Liu W, Tan M (1991) Thermochim Acta 191:135
Alves Junior S, de Almeida FV, de Sá GF, de Mello Donegá C (1997) J Lumin 72–74:478
Song YM, Xu JP, Ding L, Hou Q, Liu JW, Zhu ZL (2009) J Inorg Biochem 103:396
Arce Sagüés JA, Gillard RD, Williams PA (1979) Inorg Chim Acta 36:L411
Yuan CQ, Peng ZH, Pan QC, Li DC, Shen YF (2006) J Mol Struct 789:52
Liu YM, Peng ZH, Li DC, Zhou YH (2008) Spectrochim Acta Part A 69:471
Xie JX (1987) The application of IR spectra in organic chemistry and pharmacological chemistry. Science Press, Beijing, p 321
Yan B, Zhou B (2005) J Photochem Photobiol A Chem 171:181
Chang JH, Dong QG (2001) Spectral theory and analysis. Science Press, Beijing, p 17
Sager WF, Filipescu N, Serafin FA (1965) J Phys Chem 69:1092
Yan B, Zhang HJ, Wang SB, Ni JZ (1998) J Photochem Photobiol A Chem 116:209
Beeby A, Parker D, Williams JAG (1996) J Chem Soc Perkin Trans 1565
Hilder M, Junk PC, Kynast UH, Lezhnina MM (2009) J Photochem Photobiol A Chem 202:10
Pavithran R, Reddy MLP, Junior SA, Freire RO, Rocha GB, Lima PP (2005) Eur J Inorg Chem 2005:4129
Melhuish WH (1961) J Phys Chem 65:229
Acknowledgments
The author thanks the New Century “131” Cultivating Plan of Hangzhou City for Outstanding Young Talents for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, C. Photophysical properties of lanthanide complexes with 5-nitro-1,10-phenanthroline. Monatsh Chem 141, 631–635 (2010). https://doi.org/10.1007/s00706-010-0308-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-010-0308-2