Abstract
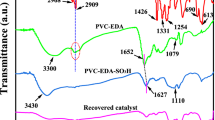
A highly sulfonated carbon as an efficient, recyclable, nontoxic, and green solid acid catalyst was readily synthesized by simultaneous sulfonation, dehydration, and carbonization of sucrose in sulfuric acid and was characterized by FT-IR, TG-DTG, XRD, and CHNS analysis, neutralization potentiometric titration, and SEM techniques. This new catalyst was used for preparation of 2-oxazolines and bis-oxazolines by reaction of β-aminoethanol with nitriles under reflux conditions. Sonication of this system enhanced the catalytic activity of the carbon-based solid acid and led to higher product yields and shorter reaction times. Another advantage of the system under ultrasonic irradiation is the ability to carry out large-scale reactions. In two cases, the catalyst was reused several times without loss of its activity.
Graphical Abstract







Similar content being viewed by others
References
Benito-Garagorri D, Bocokic V, Kirchner K (2006) Tetrahedron Lett 47:8641
Wipf P (1995) Chem Rev 95:2115
Reuman M, Meyers AI (1985) Tetrahedron 41:837
Yang D, Yip YC, Wang XC (1997) Tetrahedron Lett 38:7083
Roy RS, Gehring AM, Milne JC, Belshaw PJ, Walsh CT (1999) Nat Prod Rep 16:249
Li Q, Woods KW, Claiborne A, Gwaltney SL, Barr KJ, Liu G, Gehrke L, Credo RB, Hui YH, Lee J, Warner RB, Kovar P, Nukkala MA, Zielinski NA, Tahir SK, Fitzgerald M, Kim KH, Marsh K, Frost D, Ng SC, Rosenberg S, Sham HL (2002) Bioorg Med Chem Lett 12:465
Greene TW, Wutz PGM (1991) Protective groups in organic synthesis, 2nd edn. Wiley, New York
Fernandez AI, Fraile JM, Garcıa JI, Herrerıas CI, Mayoral JA, Salvatella L (2001) Catal Commun 2:165
Ghosh AK, Mathivanan P, Cappiello J (1998) Tetrahedron Asymmetry 9:1
Lee A, Kim W, Lee J, Hyeon T, Kim BM (2004) Tetrahedron Asymmetry 15:2595
Cwik A, Hell Z, Hegedüs A, Finta Z, Horvath Z (2002) Tetrahedron Lett 43:3985
Zhou P, Blubaum JE, Burns CT, Natale NR (1997) Tetrahedron Lett 38:7019
Clarke DS, Wood R (1996) Synth Commun 26:1335
Jnaneshwara GK, Deshpande VH, Lalithambika M, Ravindranathan T, Bedekar AV (1998) Tetrahedron Lett 39:459
Mohammadpoor-Baltork I, Khosropour AR, Hojati SF (2005) Synlett 2747
Badiang JG, Aube J (1996) J Org Chem 61:2484
Corey EJ, Ishihara K (1992) Tetrahedron Lett 33:6807
Minakata S, Nishimura M, Takahashi T, Oderaotoshi Y, Komatsu M (2001) Tetrahedron Lett 42:9019
Bower JF, Martin CJ, Rawson DJ, Slawin AMZ, Williams JMJ (1995) J Chem Soc Perkin Trans 1 2747
Witte H, Seeliger W (1974) Liebigs Ann Chem 996
Poindexter GS (1983) J Heterocycl Chem 20:1431
Pridgen LN (1982) J Org Chem 47:4319
Pirrung MC, Tumey LN (2000) J Comb Chem 2:675
George B, Papadopoulos EP (1977) J Org Chem 42:441
Bandgar BP, Pandit SS (2003) Tetrahedron Lett 44:2331
Schöning A, Debaerdemaeker T, Zander M, Friedrichsen W (1989) Chem Ber 122:1119
Kumagai T, Kawamura Y, Mukai T (1983) Tetrahedron Lett 24:2279
Chen L (2003) J Mater Sci Lett 22:953
Peng F, Zhang L, Wang H, Lv P, Yu H (2005) Carbon 43:2397
Okamura M, Takagaki A, Toda M, Kondo JN, Domen K, Tatsumi T, Hara M, Hayashi S (2006) Chem Mater 18:3039
Hara M, Yoshida T, Takagaki A, Takata T, Kondo JN, Hayashi S, Domen K (2004) Angew Chem Int Ed Engl 43:2955
Anastas PT, Kirchhoff MM (2002) Acc Chem Res 35:686
Clark JH (2002) Acc Chem Res 35:79
Okuhara T (2002) Chem Rev 102:3641
Lee JB, Park YK, Yong OB, Kang Y, Jun KW, Lee YJ, Kim HY, Lee KH, Choi WC (2006) J Power Sources 158:1251
Cano-Serrano E, Campos-Martin JM, Fierro JLG (2003) Chem Commun 246
Takagaki A, Toda M, Okamura M, Kondo JN, Hayashi S, Domen K, Hara M (2006) Catal Today 116:157
Zong MH, Duan ZQ, Lou WY, Smith TJ, Wua H (2007) Green Chem 9:434
Mohammadpoor-Baltork I, Abdollahi-Alibeik M (2003) Bull Korean Chem Soc 24:1354
Mirkhani V, Moghadam M, Tangestaninejad S, Kargar H (2006) Tetrahedron Lett 47:2129
Mirkhani V, Mohammadpoor-Baltork I, Moghadam M, Tangestaninejad S, Abdollahi-Alibeik M, Kargar H (2007) Appl Catal A 325:99
Acknowledgement
We are grateful to the Graduate Studies and Center of Excellence of Chemistry of University of Isfahan for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mirkhani, V., Moghadam, M., Tangestaninejad, S. et al. Preparation of an improved sulfonated carbon-based solid acid as a novel, efficient, and reusable catalyst for chemoselective synthesis of 2-oxazolines and bis-oxazolines. Monatsh Chem 140, 1489–1494 (2009). https://doi.org/10.1007/s00706-009-0213-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-009-0213-8