Abstract
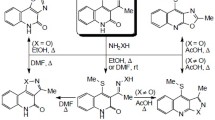
A series of ethyl 2-(substituted)-9-cyclopropyl-4-fluoro-6-oxo-1H-imidazo[4,5-h]quinoline-7-carboxylates has been prepared from ethyl 7,8-diamino-1,4-dihydroquinoline-3-carboxylate via thermally induced reactions with model alkanoic acids or via microwave-assisted cyclocondensation with some arene carboxaldehydes. Acid-catalysed hydrolysis of the resulting ester derivatives furnished the corresponding imidazoquinoline-7-carboxylic acids. The structures of these new acid and ester derivatives are based on microanalytical and spectral (IR, MS, and NMR) data.
Graphical abstract






Similar content being viewed by others
References
Abu-Sheaib ES, Zahra JA, El-Abadelah MM, Boese R (2008) Monatsh Chem (Part VI) 139 (in press)
Riether D, Mulzar J (2003) Eur J Org Chem 30
Kräutler B, Arigoni D, Golding BT (1998) Vitamin B12 and B12-proteins. Wiley-VCH, Weinheim
Woodward RB (1979) Total synthesis of vitamin B12. In: Zagalak B, Friedrich W (1979) Vitamin B12. Walter de Gruyter, Berlin
Herbert V, Das KC (1976) Vitam Horm 34:1
Preston PN (1974) Chem Rev 74:274
Grimmett MR (1984) Imidazoles and their Benzo derivatives. In: Katritsky AR, Rees CW (eds) Comprehensive heterocyclic chemistry, vol 5. Pergamon Press, Oxford, p 373
Spasov AA, Yozhitsa IN, Bugaeva LT, Anisimova VA (1999) Pharm Chem J 33:232, and refs cited therein
Hammach A, Barbosa A, Gaenzler FC, Fadra T, Goldberg D, Hao M-H, Kroe RR, Liu P, Qian KC, Ralph M, Sarko C, Soleymanzadeh F, Moss N (2006) Bioorg Med Chem Lett 16:6316
Prichard RK (1970) Nature 228:684
Friedman PA, Platzer EG (1978) Biochem Biophys Acta 544:605
Wise R, Andrews JM, Edwards LJ (1983) Antimicrob Agents Chemother 23:559
Felmingham D, O’Hare MD, Robbins MJ, Wall RA, Williams AH, Cremer AW, Ridgeway GL, Gruneberg RN (1985) Drugs Exp Clin Res 11:317
Maurer F, Grohe K (1986) Ger Offen 3, 435, 392
Maurer F, Grohe K (1986) Chem Abstr 105:97158e
Andriole VT (2000) The Quinolones. Academic Press, San Diego
Li Q, Mitscher LA, Shen LL (2000) Med Res Rev 20:231
Zhanel GG, Ennis K, Vercaigne L, Walkty A, Gin AS, Embil J, Smith H, Hoban DJ (2002) Drugs 62:13
Da Silva AD, De Almeida MV, De Souza MVN, Couri MRC (2003) Curr Med Chem 10:2
Mitscher LA (2005) Chem Rev 105:559, and refs cited therein
Glushkov RG, Dronova LN, Elina AS, Porokhovaya MV, Padeiskaya EN, Radkevich TP, Shipilova LD (1990) Khim Farmats Zhur 24:33
Glushkov RG, Dronova LN, Elina AS, Porokhovaya MV, Padeiskaya EN, Radkevich TP, Shipilova LD (1990) Chem Abstr 113:78006
Kigasawa K, Hiiragi M, Wakisaka K, Haga S, Kusama O (1980) Jpn Kokai Tokkyo Koho JP 55028920
Kigasawa K, Hiiragi M, Wakisaka K, Haga S, Kusama O (1980) Chem Abstr 93:46681
Milata V, Ilavsky D (1988) Stud Org Chem (Amesterdam) (Chem Heterocycl Compds) 35:424
Milata V, Ilavsky D (1989) Chem Abstr 110:95117
Milata V, Ilavsky D, Goljer I, Zalibera L (1994) Coll Czech Chem Commun 59:1145
Milata V, Ilavsky D, Lesko J (1988) Coll Czech Chem Commun 53:1068
Milata V, Ilavsky D (1987) Coll Czech Chem Commun 52:2918
Renault J, Chaoui M, Giogri-Renault S, Cavier R (1982) Annal Pharm Franc 40:81
Gould RG, Jacobos WA (1939) J Am Chem Soc 61:2890
Elderfield RC (1952) The chemistry of quinoline. In: Elderfield RC (ed) Heterocyclic compounds, vol 4, Chap 1. Wiley, New York, p 38
Curran TT (2005) Gould–Jacobs reactions. In: Li JJ, Corey EJ (eds) Name reactions in heterocyclic chemistry. Wiley, Hoboken, p 423
Miyamoto K, Egawa H, Fujita M, Kataoka M, Nakano J, Matsumoto J-I, Nakamura S (1989) Jpn Kokai Tokkyo Koho JP 01308281 A2
Miyamoto K, Egawa H, Fujita M, Kataoka M, Nakano J, Matsumoto J-I, Nakamura S (1990) Chem Abstr 113:23922
Fujita M, Egawa H, Miyamoto T, Nakano J, Matsumoto J-I (1996) Chem Pharm Bull 44:987
Fujita M, Egawa H, Katoka M, Miyamoto T, Nakano J, Matsumoto J-I (1995) Chem Pharm Bull 43:2123
Seman M, Belicova A, Milata V, Ilavsky D (1997) Cesk Sloven Farm 46:128
Seman M, Belicova A, Milata V, Ilavsky D (1997) Chem Abstr 127:259978
Ng RA, Lanter JC, Alford VC, Allan GF, Sbriscia T, Lundeen SG, Sui Z (2007) Bioorg Med Chem 17:1784
Ben-Alloum A, Bakkas S, Soufiaoui M (1998) Tetrahedron Lett 39:4481
Al-Hiari YM, Khanfar MA, Qaisi AM, Abu Shuheil MY, El-Abadelah MM, Boese R (2006) Heterocycles 68:1163
Abu-Sheaib ES, Zahra JA, El-Abadelah MM, Voelter W (2008) Z Naturforsch 63b:555
Zahra JA, Khanfar MA, El-Abadelah MM, Abu Thaher BA, EL-Abadla NS, Voelter W (2007) Z Naturforsch 62b:1045
Acknowledgments
We wish to thank the Deanship of Scientific Research (University of Jordan, Amman, Jordan) and DFG/Germany for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Dweik, M.R., Zahra, J.A., Khanfar, M.A. et al. Heterocycles [h]-fused to 4-oxoquinoline-3-carboxylic acid. Part VII: synthesis of some 6-oxoimidazo[4,5-h]quinoline-7-carboxylic acids and esters. Monatsh Chem 140, 221–228 (2009). https://doi.org/10.1007/s00706-008-0056-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-008-0056-8