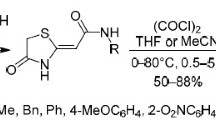
Summary.
Ethyl [4-oxo-3-(2-oxo-2-arylethyl)thiazolidin-2-ylidene]acetates and [4-oxo-3-(2-oxo-2-arylethyl)thiazolidin-2-ylidene]acetonitriles were shown to react with substituted benzaldehydes at the endocyclic methylene group leading to the corresponding 5-arylmethylidene derivatives. Their treatment with DMF · POCl3 complex yielded 3-oxo-5-aroyl-2-arylmethylidene-2,3-dihydropyrrolo[2,1-b]thiazole-7-carboxylic acids ethyl esters and -7-carbonitriles. The structures of the pyrrolothiazoles were confirmed by an X-ray crystallographic study, which indicated the (Z)-configuration at the arylmethylidene moiety.
Similar content being viewed by others
References
Wasserman HH, Suryanarayana B, Koch RC, Tse RL (1956) Chem Ind (London) 1022
HH Wasserman FM Precopio TC Liu (1952) J Am Chem Soc 74 4093 Occurrence Handle10.1021/ja01136a038
JE Baldwin MF Chan G Gallacher M Otsuka (1984) Tetrahedron 40 4513 Occurrence Handle10.1016/S0040-4020(01)98828-3
Baldwin JE, Chan MF, Gallacher G, Monk P, Prout K (1983) J Chem Soc Chem Commun 250
Maki Y, Sako M, Kurahashi N, Hirota K (1988) J Chem Soc Chem Commun 110
EM Gordon J Pluscec (1983) Tetrahedron Lett 24 3419 Occurrence Handle10.1016/S0040-4039(00)86002-5
HW Moore MJ Arnold (1983) J Org Chem 48 3365 Occurrence Handle10.1021/jo00167a056
GL Oliver JR Dann JW Gates (1958) J Am Chem Soc 80 702 Occurrence Handle10.1021/ja01536a045
IT Strukow (1952) Zh Obshch Khim 22 521
RJ Hiskey SJ Dominianni (1965) J Org Chem 30 1506
Hellstrom N, Almqvist SO, Aamisepp M, Rodmar S (1968) J Chem Soc (C) 392
JE Baldwin MA Christie (1978) J Am Chem Soc 100 4597 Occurrence Handle10.1021/ja00482a048
JE Baldwin E Lee (1986) Tetrahedron 42 6551 Occurrence Handle10.1016/S0040-4020(01)88118-7
JE Baldwin RT Freeman C Lowe CJ Schofield E Lee (1989) Tetrahedron 45 4537 Occurrence Handle10.1016/S0040-4020(01)89088-8
JE Baldwin C Lowe CJ Schofield (1986) Tetrahedron Lett 27 3461 Occurrence Handle10.1016/S0040-4039(00)84822-4
H Schafer K Gewald (1974) J Prakt Chem 316 684 Occurrence Handle10.1002/prac.19743160421
A Dondoni G Fatin M Fogagnolo A Medici (1984) Heterocycles 22 2341
Gupta AK, Ila H, Junjappa H (1988) Synthesis 284
NE Allen DB Boyd JB Campbell JB Deeter TK Elzey BJ Foster LD Hatfield JN Hobbs WJ Hornback DC Hunden ND Jones MD Kinnick JM Morin JE Munroe JK Swartzendruber DC Vogt (1989) Tetrahedron 45 1905 Occurrence Handle10.1016/S0040-4020(01)80055-7
DB Boyd TK Elzey LD Hatfield MD Kinnick JM Morin (1986) Tetrahedron Lett 27 3453 Occurrence Handle10.1016/S0040-4039(00)84820-0
EM Khalil A Pradhan WH Ojala WB Cleason RK Mishra RL Johnson (1999) J Med Chem 42 2977 Occurrence Handle10.1021/jm990140n Occurrence Handle10425107
NL Subasinghe RJ Bontems E McIntee RK Mishra RL Johnson (1993) J Med Chem 36 2356 Occurrence Handle10.1021/jm00068a013 Occurrence Handle8103113
TD Aicher B Balkan PA Bell LJ Brand SH Cheon RO Deems JB Fell WS Fillers JD Fraser J Gao DC Knorr GG Kahle CL Leone J Nadelson R Simpson HC Smith (1998) J Med Chem 41 4556 Occurrence Handle10.1021/jm9803121 Occurrence Handle9804695
T Burgemeister G Dannhardt E Graf R Obergrusberger (1987) Arch Pharm 320 799
G Dannhardt T Debaerdemaeker (1987) Arch Pharm 320 1278
DE Butler JD Leonard BW Caprathe YJ L’Italien MR Pavia FM Hershenson PH Poschel JG Marriott (1987) J Med Chem 30 498 Occurrence Handle10.1021/jm00386a010 Occurrence Handle3820221
Bespalova GV, Sedavkina VA, Ponomareva EV, Shebaldova AD, Labunskaya VI (1986) Khim Geterotsikl Soedin 1690; Chem Heterocycl Compd (Engl Transl) 22: 1370 (1986)
A Padwa ZJ Zhang (1994) Heterocycles 37 441
A Padwa LS Beall TM Heidelbaugh B Liu SM Sheehan (2000) J Org Chem 65 2684 Occurrence Handle10.1021/jo991742h Occurrence Handle10808441
Y Terao Y Yasumoto K Ikeda M Sekiya (1986) Chem Pharm Bull 34 105
AK El-Shafei AM El-Sayed H Abdel-Ghany AM El-Saghier (1990) Gazz Chim Ital 120 193
GWH Cheeseman AA Hawi (1983) J Heterocycl Chem 20 591
Coulton S, Southgate R (1992) J Chem Soc Perkin Trans 1, 961
Tverdokhlebov AV, Resnyanska EV, Tolmachev AA, Andrushko AP (2003) Synthesis 2632
Tverdokhlebov AV, Andrushko AP, Resnyanska EV, Tolmachev AA (2004) Synthesis 2317
MH Elnagdi RMH Elmoghayar AEFG Hamman SA Khallaf (1979) J Heterocycl Chem 16 1541
Satzinger G (1963) Liebigs Ann Chem 151
JL Isidor RL McKee (1973) J Org Chem 38 3615
Satzinger G (1978) Liebigs Ann Chem 473
Kambe S, Saito K, Kishi H, Hayashi T, Sakurai A (1977) Synthesis 839
Kambe S, Saito K, Sakurai A, Midorikawa H (1980) Synthesis 839
SM Sherif (1996) Monatsh Chem 127 557 Occurrence Handle10.1007/BF00807035
NS Habib SM Rida EAM Badawey HTY Fahmy HA Ghozlan (1997) Pharmazie 52 346 Occurrence Handle9183785
TS Jagodzinski A Wesolowska JG Sosniski (2001) Pol J Chem 140 422
Sheldric GM (1997) SHELXS97. Program for the Solution of Crystal Structures. University of Göttingen, Germany
Sheldric GM (1997) SHELXL97. Program for the Refinement of Crystal Structures. University of Göttingen, Germany
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tverdokhlebov, A., Andrushko, A., Tolmachev, A. et al. Synthesis of Pyrrolo[2,1-b]thiazol-3-one Derivatives. Monatsh. Chem. 136, 1781–1790 (2005). https://doi.org/10.1007/s00706-005-0356-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-005-0356-1