Summary.
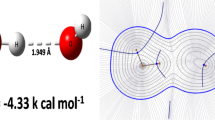
The chemical bond is a stabilization of a system with a characteristic nuclear configuration, electronic structure, and a set of physico-chemical properties. The physical origin of the chemical bond lies in an acceleration of the electrons by a joint potential of several nuclei. The quantitative description of the chemical bond in the dihydrogen molecule can be treated within the MO or VB method. Both of them have some intrinsic drawbacks which can be overcome when the MO method is followed by the configuration interaction, and the limited VB method by its complete counterpart that includes the “ionic structures”. In the end, both results are equivalent as they include the correlation energy. The amplitudes of the two-electron wave functions show that the maximum probability is obtained when the electrons are correlated – kept apart at the individual centers. This condition is very natural for the limited VB; it includes a part of the correlation energy. Therefore the VB method is a better reference for the evaluation of the exchange coupling constant that separates the ground singlet state from the lowest triplet one.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boča, R., Linert, W. Today’s View of the Chemical Bond. Monatshefte für Chemie 136, 881–923 (2005). https://doi.org/10.1007/s00706-005-0300-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-005-0300-4