Summary
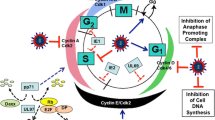
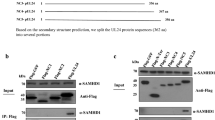
The cellular localization and virion association of the human cytomegalovirus (HCMV) UL97 protein were studied. UL97 protein demonstrated early nuclear localization followed by late perinuclear accumulation. It was found to be a structural virion constituent detected in all three enveloped forms of extracellular viral particles and shown to be phosphorylated by the virion-associated protein kinase. UL97 protein immunoprecipitated from virions and from infected cells demonstrated protein kinase activity manifested by autophosphorylation. This activity was reduced in the presence of a ganciclovir-resistance mutation at residue 460, implicated in nucleotide binding. A mutant virus, from which the proposed UL97 kinase catalytic domain had been deleted, could not be propagated in the absence of a helper wild-type virus. The characterization of UL97 protein as a virion-associated protein kinase which appears essential for viral replication, provides further insight into HCMV replication and could identify a potential novel target for antiviral therapy.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received September 2, 1997 Accepted January 14, 1998
Rights and permissions
About this article
Cite this article
Wolf, D.G., Honigman, A., Lazarovits, J. et al. Characterization of the human cytomegalovirus UL97 gene product as a virion-associated protein kinase. Arch. Virol. 143, 1223–1232 (1998). https://doi.org/10.1007/s007050050370
Published:
Issue Date:
DOI: https://doi.org/10.1007/s007050050370