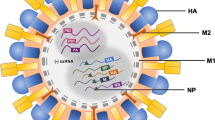
Abstract
The PI3K/Akt signalling pathway is a crucial signalling cascade that regulates transcription, protein translation, cell growth, proliferation, cell survival, and metabolism. During viral infection, viruses exploit a variety of cellular pathways, including the well-known PI3K/Akt signalling pathway. Conversely, cells rely on this pathway to stimulate an antiviral response. The PI3K/Akt pathway is manipulated by a number of viruses, including DNA and RNA viruses and retroviruses. The aim of this review is to provide up-to-date information about the role of the PI3K-Akt pathway in infection with members of five different families of negative-sense ssRNA viruses. This pathway is hijacked for viral entry, regulation of endocytosis, suppression of premature apoptosis, viral protein expression, and replication. Although less common, the PI3K/Akt pathway can be downregulated as an immunomodulatory strategy or as a mechanism for inducing autophagy. Moreover, the cell activates this pathway as an antiviral strategy for interferon and cytokine production, among other strategies. Here, we present new data concerning the role of this pathway in infection with the paramyxovirus Newcastle disease virus (NDV). Our data seem to indicate that NDV uses the PI3K/Akt pathway to delay cell death and increase cell survival as a means of improving its replication. The interference of negative-sense ssRNA viruses with this essential pathway might have implications for the development of antiviral therapies.



Similar content being viewed by others
Abbreviations
- BAD:
-
Bcl-2-associated death promoter
- FOXO:
-
Forkhead box O1 protein
- GSK3:
-
Glycogen synthase kinase 3
- INPP4B:
-
Inositol polyphosphate 4-phosphatase type II
- mTORC2:
-
Mammalian target of rapamycin complex 2
- PDK-1:
-
Phosphoinositide-dependent kinase 1
- PH:
-
Pleckstrin homology
- PHLPP:
-
PH domain leucine-rich repeat protein phosphatase
- PI3K:
-
Phosphoinositide 3-kinase
- PI(3,4)P2=PIP2:
-
Phosphatidylinositol 3,4-bisphosphate
- PI(3,4,5)P3=PIP3:
-
Phosphatidylinositol 3,4,5-trisphosphate
- PKB:
-
Protein kinase B
- PP2A:
-
Protein phosphatase 2A
- PTEN:
-
Phosphatase and tensin homologue deleted on chromosome 10
- SHIP:
-
Src homology 2 (SH2)-containing inositol 5-phosphatase
- ssRNA:
-
Single-stranded RNA
References
Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B (2010) The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 11:329–341. https://doi.org/10.1038/nrm2882
Vanhaesebroeck B, Stephens L, Hawkins P (2012) PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol 13:195–203. https://doi.org/10.1038/nrm3290
Manning BD, Toker A (2017) AKT/PKB signaling: navigating the network. Cell 169:381–405. https://doi.org/10.1016/j.cell.2017.04.001
Hawkins PT, Stephens LR (2016) Emerging evidence of signalling roles for PI(3,4)P2 in Class i and II PI3K-regulated pathways. Biochem Soc Trans 44:307–314. https://doi.org/10.1042/BST20150248
Franke TF, Kaplan DR, Cantley LC, Toker A (1997) Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science (80-) 275:665–668. https://doi.org/10.1126/science.275.5300.665
Scheid MP, Huber M, Damen JE et al (2002) Phosphatidylinositol (3,4,5)P3 is essential but not sufficient for protein kinase B (PKB) activation; phosphatidylinositol (3,4)P2 is required for PKB phosphorylation at Ser-473. Studies using cells from SH2-containing inositol-5-phosphatase knockout mice. J Biol Chem 277:9027–9035. https://doi.org/10.1074/jbc.M106755200
Bellacosa A, Chan TO, Ahmed NN et al (1998) Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene 17:313–325. https://doi.org/10.1038/sj.onc.1201947
Thomas CC, Deak M, Alessi DR, Van Aalten DMF (2002) High-resolution structure of the pleckstrin homology domain of protein kinase B/Akt bound to phosphatidylinositol (3,4,5)-trisphosphate. Curr Biol 12:1256–1262. https://doi.org/10.1016/S0960-9822(02)00972-7
Stephens L, Anderson K, Stokoe D et al (1998) Prohtein kinase B kinases that mediate phosphatidylinositol 3,4,5- trisphosphate-dependent activation of protein kinase B. Science (80-) 279:710–714. https://doi.org/10.1126/science.279.5351.710
Li H, Marshall AJ (2015) Phosphatidylinositol (3,4) bisphosphate-specific phosphatases and effector proteins: a distinct branch of PI3K signaling. Cell Signal 27:1789–1798. https://doi.org/10.1016/j.cellsig.2015.05.013
Reed DE, Shokat KM (2017) INPP4B and PTEN loss leads to PI-3,4-P2 accumulation and inhibition of PI3K in TNBC. Mol Cancer Res 15:765–775. https://doi.org/10.1158/1541-7786.MCR-16-0183
Hanada M, Feng J, Hemmings BA (2004) Structure, regulation and function of PKB/AKT—a major therapeutic target. Biochim Biophys Acta Proteins Proteomics 1697:3–16. https://doi.org/10.1016/j.bbapap.2003.11.009
Risso G, Blaustein M, Pozzi B et al (2015) Akt/PKB: one kinase, many modifications. Biochem J 468:203–214. https://doi.org/10.1042/BJ20150041
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science (80-). https://doi.org/10.1126/science.1106148
Manning BD, Cantley LC (2007) AKT/PKB Signaling: navigating downstream. Cell 129:1261–1274. https://doi.org/10.1016/j.cell.2007.06.009
Maehama T, Dixon JE (1999) PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol 9:125–128. https://doi.org/10.1016/s0962-8924(99)01519-6
Eramo MJ, Mitchell CA (2016) Regulation of PtdIns(3,4,5)P3/Akt signalling by inositol polyphosphate 5-phosphatases. Biochem Soc Trans 44:240–252. https://doi.org/10.1042/BST20150214
Dyson JM, Fedele CG, Davies EM, Becanovic J, Mitchell CA (2012) Phosphoinositide phosphatases: just as important as the kinases. Subcell Biochem 58:215–279. https://doi.org/10.1007/978-94-007-3012-0_7
Gao T, Furnari F, Newton AC (2005) PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell 18:13–24. https://doi.org/10.1016/j.molcel.2005.03.008
Kuo YC, Huang KY, Yang CH et al (2008) Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55α regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol Chem. https://doi.org/10.1074/jbc.M709585200
Diehl N, Schaal H (2013) Make yourself at home: viral hijacking of the PI3K/Akt signaling pathway. Viruses 5:3192–3212. https://doi.org/10.3390/v5123192
Cooray S (2004) The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J Gen Virol 85:1065–1076. https://doi.org/10.1099/vir.0.19771-0
Ji W-T, Liu H (2008) PI3K-Akt signaling and viral infection. Recent Pat Biotechnol. https://doi.org/10.2174/187220808786241042
Buchkovich NJ, Yu Y, Zampieri CA, Alwine JC (2008) The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat Rev Microbiol 6:266–275. https://doi.org/10.1038/nrmicro1855
Dunn EF, Connor JH (2012) HijAkt: the PI3K/Akt pathway in virus replication and pathogenesis. Prog Mol Biol Transl Sci 106:223–250. https://doi.org/10.1016/B978-0-12-396456-4.00002-X
Shaw ML, Palese P (2013) Orthomyxoviridae. In: Knipe DM, Howley PM (eds) Fields virology, 6th edn. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, pp 1151–1185
Ehrhardt C, Ludwig S (2009) A new player in a deadly game: influenza viruses and the PI3K/Akt signalling pathway. Cell Microbiol 11:863–871. https://doi.org/10.1111/j.1462-5822.2009.01309.x
Ehrhardt C, Marjuki H, Wolff T et al (2006) Bivalent role of the phosphatidylinositol-3-kinase (PI3K) during influenza virus infection and host cell defence. Cell Microbiol 8:1336–1348. https://doi.org/10.1111/j.1462-5822.2006.00713.x
Eierhoff T, Hrincius ER, Rescher U et al (2010) The epidermal growth factor receptor (EGFR) promotes uptake of influenza a viruses (IAV) into host cells. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1001099
Denisova OV, Sod̈erholm S, Virtanen S et al (2014) Akt inhibitor MK2206 prevents influenza pH1N1 virus infection in vitro. Antimicrob Agents Chemother 58:3689–3696. https://doi.org/10.1128/AAC.02798-13
Hirata N, Suizu F, Matsuda-Lennikov M et al (2014) Inhibition of Akt kinase activity suppresses entry and replication of influenza virus. Biochem Biophys Res Commun 450:891–898. https://doi.org/10.1016/j.bbrc.2014.06.077
Fujioka Y, Tsuda M, Hattori T et al (2011) The Ras-PI3K signaling pathway is involved in clathrin-independent endocytosis and the internalization of influenza viruses. PLoS One. https://doi.org/10.1371/journal.pone.0016324
Fujioka Y, Satoh AO, Horiuchi K et al (2019) A peptide derived from phosphoinositide 3-kinase inhibits endocytosis and influenza virus infection. Cell Struct Funct 44:61–74. https://doi.org/10.1247/csf.19001
Marjuki H, Gornitzky A, Marathe BM et al (2011) Influenza A virus-induced early activation of ERK and PI3K mediates V-ATPase-dependent intracellular pH change required for fusion. Cell Microbiol 13:587–601. https://doi.org/10.1111/j.1462-5822.2010.01556.x
Kuss-Duerkop SK, Wang J, Mena I et al (2017) Influenza virus differentially activates mTORC1 and mTORC2 signaling to maximize late stage replication. PLoS Pathog 13(9):e1006635
Zhirnov OP, Klenk HD (2007) Control of apoptosis in influenza virus-infected cells by up-regulation of Akt and p53 signaling. Apoptosis. https://doi.org/10.1007/s10495-007-0071-y
Shin YK, Liu Q, Tikoo SK et al (2007) Effect of the phosphatidylinositol 3-kinase/Akt pathway on influenza A virus propagation. J Gen Virol. https://doi.org/10.1099/vir.0.82483-0
Ehrhardt C, Wolff T, Pleschka S et al (2007) Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J Virol. https://doi.org/10.1128/jvi.02082-06
Shin YK, Liu Q, Tikoo SK et al (2007) Influenza A virus NS1 protein activates the phosphatidylinositol 3-kinase (PI3K)/Akt pathway by direct interaction with the p85 subunit of PI3K. J Gen Virol 88:13–18. https://doi.org/10.1099/vir.0.82419-0
Hale BG, Jackson D, Chen YH et al (2006) Influenza A virus NS1 protein binds p85β and activates phosphatidylinositol-3-kinase signaling. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.0606109103
Shin Y-K, Li Y, Liu Q et al (2007) SH3 binding motif 1 in influenza A virus NS1 protein is essential for PI3K/Akt signaling pathway activation. J Virol 81:12730–12739. https://doi.org/10.1128/jvi.01427-07
Matsuda M, Suizu F, Hirata N et al (2010) Characterization of the interaction of influenza virus NS1 with Akt. Biochem Biophys Res Commun 395:312–317. https://doi.org/10.1016/j.bbrc.2010.03.166
Jackson D, Killip MJ, Galloway CS et al (2010) Loss of function of the influenza A virus NS1 protein promotes apoptosis but this is not due to a failure to activate phosphatidylinositol 3-kinase (PI3K). Virology 396:94–105. https://doi.org/10.1016/j.virol.2009.10.004
Ayllon J, Hale BG, Garcia-Sastre A (2012) Strain-specific contribution of NS1-activated phosphoinositide 3-kinase signaling to influenza A virus replication and virulence. J Virol 86:5366–5370. https://doi.org/10.1128/jvi.06722-11
Sun Y, Li C, Shu Y et al (2012) Inhibition of autophagy ameliorates acute lung injury caused by avian influenza A H5N1 infection. Sci Signal 5:1–13. https://doi.org/10.1126/scisignal.2001931
Wang R, Zhu Y, Zhao J et al (2018) Autophagy promotes replication of influenza A virus in vitro. J Virol 93:1–17. https://doi.org/10.1128/jvi.01984-18
Hrincius ER, Dierkes R, Anhlan D et al (2011) Phosphatidylinositol-3-kinase (PI3K) is activated by influenza virus vRNA via the pathogen pattern receptor Rig-I to promote efficient type I interferon production. Cell Microbiol 13:1907–1919. https://doi.org/10.1111/j.1462-5822.2011.01680.x
Guillot L, Le Goffic R, Bloch S et al (2005) Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem 280:5571–5580. https://doi.org/10.1074/jbc.M410592200
Lamb RA, Parks GD (2013) Paramyxoviridae. In: Knipe DM, Howley PM (eds) Fields virology, 6th edn. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, pp 957–995
Peters K, Chattopadhyay S, Sen GC (2008) IRF-3 activation by sendai virus infection is required for cellular apoptosis and avoidance of persistence. J Virol 82:3500–3508. https://doi.org/10.1128/jvi.02536-07
White CL, Chattopadhyay S, Sen GC (2011) Phosphatidylinositol 3-kinase signaling delays sendai virus-induced apoptosis by preventing XIAP degradation. J Virol 85:5224–5227. https://doi.org/10.1128/jvi.00053-11
Kang Y, Yuan R, Zhao X et al (2017) Transient activation of the PI3K/Akt pathway promotes Newcastle disease virus replication and enhances anti-apoptotic signaling responses. Oncotarget 8:23551–23563. https://doi.org/10.18632/oncotarget.15796
Bai Y, Chen Y, Hong X et al (2018) Newcastle disease virus enhances the growth-inhibiting and proapoptotic effects of temozolomide on glioblastoma cells in vitro and in vivo. Sci Rep 8:1–12. https://doi.org/10.1038/s41598-018-29929-y
Sun M, Fuentes SM, Timani K et al (2008) Akt plays a critical role in replication of nonsegmented negative-stranded RNA viruses. J Virol 82:105–114. https://doi.org/10.1128/jvi.01520-07
Yeon SH, Song MJ, Kang HR, Lee JY (2015) Phosphatidylinositol-3-kinase and Akt are required for RIG-I-mediated anti-viral signalling through cross-talk with IPS-1. Immunology 144:312–320. https://doi.org/10.1111/imm.12373
Luthra P, Sun D, Wolfgang M, He B (2008) AKT1-dependent activation of NF-B by the L protein of parainfluenza virus 5. J Virol 82:10887–10895. https://doi.org/10.1128/jvi.00806-08
Sharma NR, Mani P, Nandwani N et al (2010) Reciprocal regulation of AKT and MAP kinase dictates virus-host cell fusion. J Virol 84:4366–4382. https://doi.org/10.1128/jvi.01940-09
Avota E, Avots A, Niewiesk S et al (2001) Disruption of Akt kinase activation is important for immunosuppression induced by measles virus. Nat Med 7:725–731. https://doi.org/10.1038/89106
Carsillo M, Kim D, Niewiesk S (2010) Role of AKT kinase in measles virus replication. J Virol 84:2180–2183. https://doi.org/10.1128/jvi.01316-09
Avota E, Muller N, Klett M, Schneider-Schaulies S (2004) Measles virus interacts with and alters signal transduction in T-cell lipid rafts. J Virol 78:9552–9559. https://doi.org/10.1128/jvi.78.17.9552-9559.2004
Thomas KW, Monick MM, Staber JM et al (2002) Respiratory syncytial virus inhibits apoptosis and induces NF-κB activity through a phosphatidylinositol 3-kinase-dependent pathway. J Biol Chem 277:492–501. https://doi.org/10.1074/jbc.M108107200
Monick MM, Cameron K, Powers LS et al (2004) Sphingosine kinase mediates activation of extracellular signal-related kinase and Akt by respiratory syncytial virus. Am J Respir Cell Mol Biol 30:844–852. https://doi.org/10.1165/rcmb.2003-0424OC
Lindemans CA, Coffer PJ, Schellens IMM et al (2006) Respiratory syncytial virus inhibits granulocyte apoptosis through a phosphatidylinositol 3-kinase and NF-κB-dependent mechanism. J Immunol 176:5529–5537. https://doi.org/10.4049/jimmunol.176.9.5529
Bitko V, Shulyayeva O, Mazumder B et al (2007) Nonstructural proteins of respiratory syncytial virus suppress premature apoptosis by an NF- B-dependent, interferon-independent mechanism and facilitate virus growth. J Virol 81:1786–1795. https://doi.org/10.1128/jvi.01420-06
Dunn EF, Fearns R, Connor JH (2009) Akt inhibitor Akt-IV blocks virus replication through an Akt-independent mechanism. J Virol 83:11665–11672. https://doi.org/10.1128/jvi.01092-09
Machado D, Pizzorno A, Hoffmann J et al (2018) Role of p53/NF-кB functional balance in respiratory syncytial virus-induced inflammation response. J Gen Virol 99:489–500. https://doi.org/10.1099/jgv.0.001040
Groskreutz DJ, Monick MM, Yarovinsky TO et al (2007) Respiratory syncytial virus decreases p53 protein to prolong survival of airway epithelial cells. J Immunol 179:2741–2747. https://doi.org/10.4049/jimmunol.179.5.2741
Muraro SP, De Souza GF, Gallo SW et al (2018) Respiratory syncytial virus induces the classical ROS-dependent NETosis through PAD-4 and necroptosis pathways activation. Sci Rep 8:1–12. https://doi.org/10.1038/s41598-018-32576-y
Lyles DS, Kuzmin IV, Rupprecht CE (2013) Rhabdoviridae. In: Knipe DM, Howley PM (eds) Fields virology, 6th edn. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, pp 885–922
Minami K, Tambe Y, Watanabe R et al (2007) Suppression of viral replication by stress-inducible GADD34 Protein via the mammalian serine/threonine protein kinase mTOR pathway. J Virol 81:11106–11115. https://doi.org/10.1128/jvi.01063-07
Saeed MF, Kolokoltsov AA, Freiberg AN et al (2008) Phosphoinositide-3 kinase-akt pathway controls cellular entry of ebola virus. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1000141
Cheng CY, Huang WR, Chi PI et al (2015) Cell entry of bovine ephemeral fever virus requires activation of Src-JNK-AP1 and PI3K-Akt-NF-κB pathways as well as Cox-2-mediated PGE2/EP receptor signalling to enhance clathrin-mediated virus endocytosis. Cell Microbiol 17:967–987. https://doi.org/10.1111/cmi.12414
Dunn EF, Connor JH (2011) Dominant inhibition of Akt/protein kinase B signaling by the matrix protein of a negative-strand RNA virus. J Virol 85:422–431. https://doi.org/10.1128/jvi.01671-10
Schabbauer G, Luyendyk J, Crozat K et al (2008) TLR4/CD14-mediated PI3K activation is an essential component of interferon-dependent VSV resistance in macrophages. Mol Immunol 45:2790–2796. https://doi.org/10.1016/j.molimm.2008.02.001
Shelly S, Lukinova N, Bambina S et al (2009) Autophagy is an essential component of drosophila immunity against vesicular stomatitis virus. Immunity 30:588–598. https://doi.org/10.1016/j.immuni.2009.02.009
Liu J, Wang H, Gu J et al (2017) BECN1-dependent CASP2 incomplete autophagy induction by binding to rabies virus phosphoprotein. Autophagy 13:739–753. https://doi.org/10.1080/15548627.2017.1280220
Feldmann H, Sanchez A, Geisbert TW (2013) Filoviridae: Marburg and ebola viruses. In: Fields virology, 6th edn
Nanbo A, Imai M, Watanabe S et al (2010) Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1001121
Stark A, Sriskantharajah S, Hessel EM, Okkenhaug K (2015) PI3K inhibitors in inflammation, autoimmunity and cancer. Curr Opin Pharmacol 23:82–91. https://doi.org/10.1016/j.coph.2015.05.017.PI3K
Hsu ACY, Starkey MR, Hanish I et al (2015) Targeting PI3K-p110α suppresses influenza virus infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 191:1012–1023. https://doi.org/10.1164/rccm.201501-0188OC
Fujita A, Kan-o K, Tonai K et al (2020) Inhibition of PI3Kδ enhances poly I:C-induced antiviral responses and inhibits replication of human metapneumovirus in murine lungs and human bronchial epithelial cells. Front Immunol 11:1–19. https://doi.org/10.3389/fimmu.2020.00432
Nayak MK, Agrawal AS, Bose S et al (2014) Antiviral activity of baicalin against influenza virus H1N1-pdm09 is due to modulation of NS1-mediated cellular innate immune responses. J Antimicrob Chemother 69:1298–1310. https://doi.org/10.1093/jac/dkt534
Sun Q, Wu R, Cai S et al (2011) Synthesis and biological evaluation of analogues of AKT (protein kinase B) inhibitor-IV. J Med Chem 54:1126–1139. https://doi.org/10.1021/jm100912b
Murray JL, McDonald NJ, Sheng J et al (2012) Inhibition of influenza A virus replication by antagonism of a PI3K-AKT-mTOR pathway member identified by gene-trap insertional mutagenesis. Antivir Chem Chemother 22:205–215. https://doi.org/10.3851/IMP2080
Smallwood HS, Duan S, Morfouace M et al (2017) Targeting metabolic reprogramming by influenza infection for therapeutic intervention. Cell Rep 19:1640–1653. https://doi.org/10.1016/j.celrep.2017.04.039
Chen WC, Liu L, Shen YF et al (2018) A new coumarin derivative plays a role in rhabdoviral clearance by interfering glycoprotein function during the early stage of viral infection. Cell Signal 51:199–210. https://doi.org/10.1016/j.cellsig.2018.08.007
Richart SM, Li YL, Mizushina Y et al (2018) Synergic effect of curcumin and its structural analogue (Monoacetylcurcumin) on anti-influenza virus infection. J Food Drug Anal 26:1015–1023. https://doi.org/10.1016/j.jfda.2017.12.006
Li YC, Peng SZ, Chen HM et al (2012) Oral administration of patchouli alcohol isolated from Pogostemonis Herba augments protection against influenza viral infection in mice. Int Immunopharmacol 12:294–301. https://doi.org/10.1016/j.intimp.2011.12.007
Yu Y, Zhang Y, Wang S et al (2019) Inhibition effects of patchouli alcohol against influenza a virus through targeting cellular PI3K/Akt and ERK/MAPK signaling pathways. Virol J 16:1–16. https://doi.org/10.1186/s12985-019-1266-x
Wu MS, Yen HR, Chang CW et al (2011) Mechanism of action of the suppression of influenza virus replication by Ko-Ken Tang through inhibition of the phosphatidylinositol 3-kinase/Akt signaling pathway and viral RNP nuclear export. J Ethnopharmacol 134:614–623. https://doi.org/10.1016/j.jep.2011.01.005
Wang QW, Su Y, Sheng JT et al (2018) Anti-influenza A virus activity of rhein through regulating oxidative stress, TLR4, Akt, MAPK, and NF-κB signal pathways. PLoS One 13:1–19. https://doi.org/10.1371/journal.pone.0191793
Acknowledgements
We thank Dr. Adolfo García-Sastre for providing recombinant rNDV-F3aa-mRFP, NDV, and polyclonal anti-NDV antibodies. We also thank Emma Keck for revising the English.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Bert K. Rima.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Blanco, J., Cameirao, C., López, M.C. et al. Phosphatidylinositol-3-kinase-Akt pathway in negative-stranded RNA virus infection: a minireview. Arch Virol 165, 2165–2176 (2020). https://doi.org/10.1007/s00705-020-04740-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-020-04740-1