Abstract
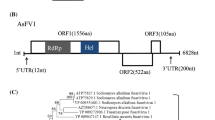
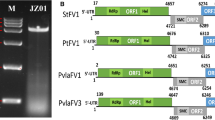
A novel virus, Botryosphaeria dothidea fusarivirus 1 (BdFV1), was isolated from a fungal strain, SDAU11-86 of Botryosphaeria dothidea, and its complete genome sequence was determined. BdFV1 has a single-stranded positive-sense (+ssRNA) genome with 6,179 nucleotides, excluding the poly(A) tail. The genome of BdFV1 contains two putative open reading frames (ORFs). The first ORF encodes a large polyprotein of 1,544 amino acids (aa) with conserved RNA-dependent RNA polymerase and viral helicase domains. The second ORF encodes a putative 481-aa protein with unknown function. Sequence comparisons and phylogenetic analysis suggested that BdFV1 is a novel mycovirus belonging to the newly proposed family “Fusariviridae”. This is the first report of a +ssRNA mycovirus in B. dothidea.


Similar content being viewed by others
References
Ghabrial SA, Castón JR, Jiang D et al (2015) 50-plus years of fungal viruses. Virology 479:356–368
Xie J, Jiang D (2014) New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Ann Rev Phytopathol 52:45–68
Donaire L, Pagán I, Ayllón MA (2016) Characterization of Botrytis cinerea negative-stranded RNA virus 1, a new mycovirus related to plant viruses, and a reconstruction of host pattern evolution in negative-sense ssRNA viruses. Virology 499:212–218
Liu L, Xie J, Cheng J et al (2014) Fungal negative-stranded RNA virus that is related to bornaviruses and nyaviruses. Proc Natl Acad Sci 111(33):12205–12210
Marzano SYL, Nelson BD, Ajayi-Oyetunde O et al (2016) Identification of diverse mycoviruses through metatranscriptomics characterization of the viromes of five major fungal plant pathogens. J Virol 90(15):6846–6863
Mu F, Xie J, Cheng S et al (2018) Virome characterization of a collection of S. sclerotiorum from Australia. Front Microbiol 8:2540
Wang L, He H, Wang S et al (2018) Evidence for a novel negative-stranded RNA mycovirus isolated from the plant pathogenic fungus Fusarium graminearum. Virology 518:232–240
Yu X, Li B, Fu Y et al (2010) A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus. Proc Natl Acad Sci 107(18):8387–8392
King AMQ et al (2011) Virus taxonomy: ninth report of the International Committee on Taxonomy of viruses, vol 9. Elsevier, Amsterdam
Li K, Zheng D, Cheng J et al (2016) Characterization of a novel Sclerotinia sclerotiorum RNA virus as the prototype of a new proposed family within the order Tymovirales. Virus Res 219:92–99
Hamid M, Xie J, Wu S et al (2018) A novel deltaflexivirus that infects the plant fungal pathogen, Sclerotinia sclerotiorum, can be transmitted among host vegetative incompatible strains. Viruses. 10(6):295
Zhang R, Liu S, Chiba S et al (2014) A novel single-stranded RNA virus isolated from a phytopathogenic filamentous fungus, Rosellinia necatrix, with similarity to hypo-like viruses. Front Microbiol 5:360
Picarelli MASC, Forgia M, Rivas EB et al (2019) Extreme diversity of mycoviruses present in isolates of Rhizoctonia solani AG2-2 LP from Zoysia japonica from Brazil. Fronti Cell Infect Microbiol 9:244
Dissanayake AJ, Phillips AJL, Li XH et al (2016) Botryosphaeriaceae: current status of genera and species. Mycosphere. 7(7):1001–1073
Marsberg A, Kemler M, Jami F et al (2017) Botryosphaeria dothidea: a latent pathogen of global importance to woody plant health. Mol Plant Pathol 18(4):477–488
Tang W, Ding Z, Zhou ZQ et al (2012) Phylogenetic and pathogenic analyses show that the causal agent of apple ring rot in China is Botryosphaeria dothidea. Plant Dis 96(4):486–496
Xu C, Wang C, Ju L et al (2015) Multiple locus genealogies and phenotypic characters reappraise the causal agents of apple ring rot in China. Fungal Divers 71(1):215–231
Zhai L, Zhang M, Lv G et al (2014) Biological and molecular characterization of four Botryosphaeria species isolated from pear plants showing stem wart and stem canker in China. Plant Dis 98(6):716–726
Yan JY, Xie Y, Zhang W et al (2013) Species of Botryosphaeriaceae involved in grapevine dieback in China. Fungal Divers 61(1):221–236
Liu X, Liu HX, Han XL et al (2018) First Report of Botryosphaeria dothidea causing fruit rot of Rellowhorn (Xanthoceras sorbifolium) in China. Plant Dis 102(8):1662
Liu HX, Liang J, Zhao JP et al (2007) Study on the etiology of pomegranate canker diseases. Sci Silvae Sin 04:54–58
Zhang YL, Liu HX, Xu YY et al (2018) The tea brown blight disease caused by co-infection of Glomerella cingulata f. sp. camelliae and Botryosphaeria dothidea. J Tea Sci 38(01):87–93
Kang L, Hao H, Yang Z et al (2009) The advances in the research of apple ring rot. Chin Agric Sci Bull 25(09):188–191 (in Chinese)
Wang LP, Jiang JJ, Wang YF et al (2014) Hypovirulence of the phytopathogenic fungus Botryosphaeria dothidea: association with a coinfecting chrysovirus and a partitivirus. J Virol 88(13):7517–7527
Zhai L, Xiang J, Zhang M et al (2016) Characterization of a novel double-stranded RNA mycovirus conferring hypovirulence from the phytopathogenic fungus Botryosphaeria dothidea. Virology. 493:75–85
Ding Z, Zhou T, Guo LY (2017) Characterization of a novel strain of Botryosphaeria dothidea chrysovirus 1 from the apple white rot pathogen Botryosphaeria dothidea. Arch Virol 162(7):2097–2102
Zhai L, Hong N, Zhang M et al (2015) Complete dsRNA sequence of a novel victorivirus isolated from the pear stem wart fungus Botryosphaeria dothidea. Arch Virol 160(2):613–616
Zhai L, Yang M, Zhang M et al (2019) Characterization of a Botybirnavirus conferring hypovirulence in the phytopathogenic fungus Botryosphaeria dothidea. Viruses. 11(3):266
Marais A, Nivault A, Faure C et al (2018) Molecular characterization of a novel fusarivirus infecting the plant-pathogenic fungus Neofusicoccum luteum. Arch Virol 163(2):559–562
Choi YG, Randles JW (1997) Microgranular cellulose improves dsRNA recovery from plant nucleic acid extracts. BioTechniques. 23(4):610–611
Cock PJA, Fields CJ, Goto N et al (2009) The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucleic Acids Res 38(6):1767–1771
Hansen KD, Brenner SE, Dudoit S (2010) Biases in Illumina transcriptome sequencing caused by random hexamer priming. Nucleic Acids Res 38(12):e131–e131
Jiang L, Schlesinger F, Davis CA et al (2011) Synthetic spike-in standards for RNA-seq experiments. Genome Res 21(9):1543–1551
Lambden PR, Cooke SJ, Caul EO et al (1992) Cloning of noncultivatable human rotavirus by single primer amplification. J Virol 66(3):1817–1822
Acknowledgements
The authors are grateful to Dr. Huanting Liu (University of St Andrews, UK) for help in improving the manuscript.
Funding
This research was supported by the National Key R&D Program of China (2017YFD0600102-7 and 2017YFD0201103), the National Natural Science Foundation of China (31770684), and the Natural Science Foundation of Shandong Province (ZR2017MC042).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Ioly Kotta-Loizou.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, W., Hai, D., Mu, F. et al. Molecular characterization of a novel fusarivirus infecting the plant-pathogenic fungus Botryosphaeria dothidea. Arch Virol 165, 1033–1037 (2020). https://doi.org/10.1007/s00705-020-04554-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-020-04554-1