Abstract
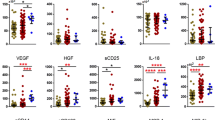
There is no definitive predictor of dengue severity, and this has led to a very large number of unnecessary hospitalizations worldwide. Although mast cell mediators are believed to a play role in dengue severity, the lack of precise kinetic data demands further research on early predictors. We enrolled 111 patients with confirmed dengue and 85 with “other febrile illness” (OFI) in a hospital-based prospective study in Vietnam. Dengue patients were classified as level 1, 2, or 3 based on the clinical intervention received. Blood samples were collected from each patient every day (pre- and post-defervescence) and after discharge. Plasma chymase, total IgE, and dengue-specific IgE were measured. Dengue-specific IgE levels showed an increasing trend during the course of illness and remained high even at post-discharge, although no significant difference was observed among severity levels. Total IgE showed no such trend. The specific IgE/total IgE ratio (S/T ratio) remained constantly higher in level 3 patients compared to other levels, with a significant difference at some time points. The S/T ratio of acute phase samples (before defervescence) tended to increase with increasing severity (level 1 < 2 < 3), and was significantly higher in level 3 patients than in level 1 and OFI patients. As an early predictor of severity allowing level 3 patients to be distinguished from other dengue patients, the S/T ratio achieved a sensitivity of 75% and specificity of 68%. We describe the kinetic profiles of IgEs, their ratio, and chymase levels at different severity levels. The S/T ratio was found to be associated with dengue severity, suggesting that it could potentially be used as an early predictor of severity.





Similar content being viewed by others
References
Abraham SN, St John AL (2010) Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol 10:440–452
Alexander N, Balmaseda A, Coelho ICB, Dimaano E, Hien TT, Hung NT, Jänisch T, Kroeger A, Lum LCS, Martinez E, Siqueira JB, Thuy TT, Villalobos I, Villegas E, Wills B, European Union WHOsDSG (2011) Multicentre prospective study on dengue classification in four South-east Asian and three Latin American countries. Trop Med Int Health 16:936–948
Bachal R, Alagarasu K, Singh A, Salunke A, Shah P, Cecilia D (2015) Higher levels of dengue-virus-specific IgG and IgA during pre-defervescence associated with primary dengue hemorrhagic fever. Arch Virol 160:2435–2443
Carrasco LR, Leo YS, Cook AR, Lee VJ, Thein TL, Go CJ, Lye DC (2014) Predictive tools for severe dengue conforming to World Health Organization 2009 criteria. PLoS Negl Trop Dis 8:e2972
da Silva EZM, Jamur MC, Oliver C (2014) Mast cell function. J Histochem Cytochem 62:698–738
Di Lorenzo G, Mansueto P, Pacor ML, Rizzo M, Castello F, Martinelli N, Ditta V, Lo Bianco C, Leto-Barone MS, D’Alcamo A, Di Fede G, Rini GB, Ditto AM (2009) Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J Allergy Clin Immunol 123(1103–1110):e1104
Furuta T, Murao LA, Lan NTP, Huy NT, Huong VTQ, Thuy TT, Tham VD, Nga CTP, Ha TTN, Ohmoto Y, Kikuchi M, Morita K, Yasunami M, Hirayama K, Watanabe N (2012) Association of mast cell-Derived VEGF and proteases in dengue shock syndrome. PLOS Negl Trop Dis 6:e1505
Godoi IP, Lemos LL, de Araujo VE, Bonoto BC, Godman B, Guerra Junior AA (2017) CYD-TDV dengue vaccine: systematic review and meta-analysis of efficacy, immunogenicity and safety. J Comp Eff Res 6:165–180
Innis BL, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, Puttisri P, Hoke CH (1989) An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg 40:418–427
Jacqueline L, Deen EH, Wills Bridget, Balmaseda Angel, Hammond Samantha Nadia, Rocha Crisanta, Dung Nguyen Minh, Hung Nguyen Thanh, Hien Tran Tinh, Farrar Jeremy J (2006) The WHO dengue classification and case definitions: time for a reassessment. Lancet 368:170–173
John ALS, Rathore APS, Yap H, Ng M-L, Metcalfe DD, Vasudevan SG, Abraham SN (2011) Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. PNAS 108:9190–9195
John ALS (2013) Influence of mast cells on dengue protective immunity and immune pathology. PLOS Pathog 9:e1003783
King CA, Anderson R, Marshall JS (2002) Dengue virus selectively induces human mast cell chemokine production. J Virol 76:8408–8419
Koraka P, Burghoorn-Maas CP, Falconar A, Setiati TE, Djamiatun K, Groen J, Osterhaus ADME (2003) Detection of immune-complex-dissociated nonstructural-1 antigen in patients with acute dengue virus infections. J Clin Microbiol 41:4154–4159
Koraka P, Murgue B, Deparis X, Setiati TE, Suharti C, van Gorp ECM, Ce Hack, Osterhaus ADME, Groen J (2003) Elevated levels of total and dengue virus-specific immunoglobulin E in patients with varying disease severity. J Med Virol 70:91–98
Koraka P (2007) Dengue virus specific immune response: implications for laboratory diagnosis and vaccine development
Krystel-Whittemore M, Dileepan KN, Wood JG (2016) Mast cell: a multi-functional master cell. Front Immunol 6
Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV (1992) Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol 30:545–551
Mabalirajan U, Kadhiravan T, Sharma SK, Banga A, Ghosh B (2005) Short report: TH2 immune response in patients with dengue during defervescence: Preliminary evidence. Am J Trop Med Hyg 72:783–785
Narvaez F, Gutierrez G, Pérez MA, Elizondo D, Nuñez A, Balmaseda A, Harris E (2011) Evaluation of the traditional and revised WHO classifications of dengue disease severity. PLOS Neglected Tropical Diseases 5
Nguyen MT, Ho TN, Nguyen VVC, Nguyen TH, Ha MT, Ta VT, Nguyen LDH, Phan L, Han KQ, Duong THK, Tran NBC, Wills B, Wolbers M, Simmons CP (2017) An evidence-based algorithm for early prognosis of severe dengue in the outpatient setting. Clin Infect Dis 64:656–663
Ngwe Tun MM, Thant KZ, Inoue S, Kurosawa Y, Lwin YY, Lin S, Aye KT, Thet Khin P, Myint T, Htwe K, Mapua CA, Natividad FF, Hirayama K, Morita K (2013) Serological characterization of dengue virus infections observed among dengue hemorrhagic fever/dengue shock syndrome cases in upper Myanmar. J Med Virol 85:1258–1266
OhAinle M, Balmaseda A, Macalalad AR, Tellez Y, Zody MC, Saborio S, Nunez A, Lennon NJ, Birren BW, Gordon A, Henn MR, Harris E (2011) Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci Transl Med 3:114ra128
Potts JA, Gibbons RV, Rothman AL, Srikiatkhachorn A, Thomas SJ, P-o Supradish, Lemon SC, Libraty DH, Green S, Kalayanarooj S (2010) Prediction of dengue disease severity among pediatric Thai patients using early clinical laboratory indicators. PLOS Neglected Tropical Diseases 4:e769
Sanchez LF, Hotta H, Hotta S, Homma M (1986) Degranulation and histamine release from murine mast cells sensitized with dengue virus-immune sera. Microbiol Immunol 30:753–759
Simmons CP, McPherson K, Van Vinh Chau N, Hoai Tam DT, Young P, Mackenzie J, Wills B (2015) Recent advances in dengue pathogenesis and clinical management. Vaccine 33:7061–7068
St John AL, Rathore APS, Raghavan B, Ng M-L, Abraham SN (2013) Contributions of mast cells and vasoactive products, leukotrienes and chymase, to dengue virus-induced vascular leakage. eLife 2
St. John AL (2013) Influence of mast cells on dengue protective immunity and immune pathology. PLOS Pathogens 9
Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, Hay SI, Bedi N, Bensenor IM, Castaneda-Orjuela CA, Chuang TW, Gibney KB, Memish ZA, Rafay A, Ukwaja KN, Yonemoto N, Murray CJ (2016) The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis 16:712–723
Tamibmaniam J, Hussin N, Cheah WK, Ng KS, Muninathan P (2016) Proposal of a clinical decision tree algorithm using factors associated with severe dengue infection. PLOS One 11:e0161696
Vázquez S, Cabezas S, Pérez AB, Pupo M, Ruiz D, Calzada N, Bernardo L, Castro O, González D, Serrano T, Sanchez A, Guzmán MG (2007) Kinetics of antibodies in sera, saliva, and urine samples from adult patients with primary or secondary dengue 3 virus infections. Int J Infect Dis 11:256–262
Vultaggio A, Virgili G, Gaeta F, Romano A, Maggi E, Matucci A (2015) High serum β-lactams specific/total IgE ratio is associated with immediate reactions to β-lactams antibiotics. PLOS One 10
WHO (1997) Dengue haemorrhagic fever: diagnosis, treatment, prevention and control, 2nd edn. World Health Organization (WHO), Geneva
WHO (2009) Dengue guidelines for diagnosis, treatment, prevention and control: new edition. World Health Organization (WHO), Geneva
Acknowledgments
The authors would like to thank all of the participants who generously enrolled in this study. This study was conducted (in part) at the Joint Usage/Research Center on Tropical Disease, Institute of Tropical Medicine, Nagasaki University, Japan.
Funding
This work was supported by Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) (Grant Number 16fm0108001h0002 to KH).
Author information
Authors and Affiliations
Contributions
MI, SPD, LNW, VTQH, KM, NTH, KH conceived and designed the study. MI, SPD, MMNT, LHP, NVT, TVA, LNW, VTQH performed the experiments and collected samples. MI, SPD, SM, DHM, NTH, KH analyzed and interpreted the data. SPD, MI, NTH, KH wrote the draft. All authors contributed to the writing and revisions and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
Ethical approval for this study was obtained from the Institutional Review Boards (IRBs) of the Pasteur Institute in Ho Chi Minh City, Vietnam (No. 602/ QD-Pas 27/12/10) and the Institute of Tropical Medicine, Nagasaki University, Japan (No. 11063072). All procedures performed in studies involving human participants were in accordance with the ethical standards of the IRBs and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All of the participants were duly informed about the study, and written informed consent was obtained from the study participants or their guardians/parents (in the case of minors) prior to their enrolment in the study.
Additional information
Handling Editor: Chan-Shing Lin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Inokuchi, M., Dumre, S.P., Mizukami, S. et al. Association between dengue severity and plasma levels of dengue-specific IgE and chymase. Arch Virol 163, 2337–2347 (2018). https://doi.org/10.1007/s00705-018-3849-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-3849-z