Abstract
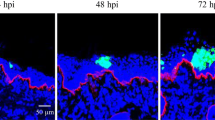
The respiratory mucosa is the common port of entry of equine herpesvirus type 1 (EHV-1) and several other alphaherpesviruses. An important prerequisite for successful host invasion of the virus is to cross the epithelial cell layer and the underlying basement membrane barrier. In the present study, an analysis was performed to see if an EHV-1 infection of nasal mucosa epithelial cells leads to damage of the underlying extracellular matrix proteins. Nasal mucosa explants were inoculated with EHV-1 and collected at 0, 24 and 48 hours post-inoculation (hpi). Then, double immunofluorescence staining was performed to detect viral-antigen-positive cells on the one hand and integrin alpha 6, laminin, collagen IV and collagen VII on the other hand. The area of these extracellular matrix proteins was measured in regions of interest (ROIs) at a magnification of 200X by means of the software imaging system ImageJ. ROIs were defined beneath uninfected and infected regions. In uninfected regions, 22-28 % of the ROI was stained for integrin alpha 6, 18-37 % for laminin, 14-38 % for collagen IV and 18-26 % for collagen VII. In infected regions, the percentage positive for integrin alpha 6 was significantly decreased to 0.1-9 % and 0.1-6 % after 24 and 48 hours of inoculation, respectively. Infection did not alter the percentages for laminin and collagen IV. For collagen VII, an increase in the percentage (from 18-26 % to 28-39 %) could be observed underneath EHV-1-infected plaques at 48 hours of inoculation. In conclusion, the results revealed a substantial impact of EHV-1 infection on integrin alpha 6 and collagen VII, two important components of the extracellular matrix, which are associated with the basement membrane and may facilitate virus penetration via hijacked leukocytes to underlying tissues.




Similar content being viewed by others
References
Allen GP, Kydd JH, Slater JD, Smith KC (2004) Equid herpesvirus 1 and equid herpesvirus 4 infections. In: Coetzer JAW, Thomson GR, Tustin RC (eds) Infectious diseases of livestock, vol 2, 1st edn. OUP, Newmarket, pp 829–859
Bannazadeh Baghi H, Lavel K, Favoreel H, Nauwynck HJ (2014) Isolation and characterization of equine nasal mucosal CD172a+ cells. Vet Immunol Immunopathol 157:155–163
Bannazadeh Baghi H, Nauwynck HJ (2014) Impact of equine herpesvirus type 1 (EHV-1) infection on the migration of monocytic cells through equine nasal mucosa. Comp Immunol Microbiol Infect Dis 37:321–329
Borradori L, Sonnenberg A (1999) Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol 112:411–418
Brittingham R, Uitto J, Fertala A (2006) High-affinity binding of the NC1 domain of collagen VII to laminin 5 and collagen IV. Biochem Biophys Res Commun 343:3692–3699
Bürki F, Rossmanith W, Nowotny N, Pallan C, Mostl K, Lussy H (1990) Viremia and abortions are not prevented by two commercial equine herpesvirus-1 vaccines after experimental challenge of horses. Vet Q 12:80–86
Chen M, Marinkovich MP, Veis A, O’Toole EA, Rao CN, Cai XY, Woodley DT (1997) Interactions of the amino-terminal noncollagenous (NC1) domain of type VII collagen with extracellular matrix components: a potential role in epidermal-dermal adherence in human skin. J Biol Chem 272:14516–14522
Dunowska M (2014) A review of equid herpesvirus 1 for the veterinary practitioner. Part B: pathogenesis and epidemiology. N Z Vet J 62:179–188
Erickson AC, Couchman JR (2000) Still more complexity in mammalian basement membranes. J Histochem Cytochem 48:1291–1306
Evans MJ, Fanucchi MV, Miller LA, Carlson MA, Nishio SJ, Hyde DM (2010) Reduction of collagen vii anchoring fibrils in the airway basement membrane zone of infant rhesus monkeys exposed to house dust mite. Am J Physiol Lung Cell Mol Physiol 298:543–547
Evans MJ, Fanucchi MV, Plopper CG, Hyde DM (2010) Postnatal development of the lamina reticularis in primate airways. Anat Rec 293:947–954
Evans MJ, Cox RA, Shami SG, Plopper CG (1990) Junctional adhesion mechanisms in airway basal cells. Am J Respir Cell Mol Biol 3:341–347
Garré B, Gryspeerdt A, Croubels S, De Backer P, Nauwynck H (2009) Evaluation of orally administered valacyclovir in experimentally EHV1-infected ponies. Vet Microbiol 135:214–221
Glorieux S, Van den Broeck W, van der Meulen KM, Van Reeth K, Favoreel HW, Nauwynck HJ (2007) In vitro culture of porcine respiratory nasal mucosa explants for studying the interaction of porcine viruses with the respiratory tract. J Virol Methods 142:105–112
Glorieux S, Bachert C, Favoreel HW, Vandekerckhove AP, Steukers L, Rekecki A, Van den Broeck W, Goossens J, Croubels S, Clayton RF, Nauwynck HJ (2011) Herpes simplex virus type 1 penetrates the basement membrane in human nasal respiratory mucosa. PLoS One 6:e22160
Gryspeerdt AC, Vandekerckhove AP, Garré B, Barbé F, Van de Walle GR, Nauwynck HJ (2010) Differences in replication kinetics and cell tropism between neurovirulent and non-neurovirulent EHV1 strains during the acute phase of infection in horses. Vet Microbiol 142:242–253
Kajiji S, Tamura RN, Quaranta V (1989) A novel integrin (aE b4) from human epithelial cells suggests a fourth family of integrin adhesion receptors. EMBO J 8:673–680
Kalluri R (2003) Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 3:422–433
Kelley LC, Lohmer LL, Hagedorn EJ, Sherwood DR (2014) Traversing the basement membrane in vivo: a diversity of strategies. J Cell Biol 204:291–302
Kydd JH, Smith KC, Hannant D, Livesay GJ, Mumford JA (1994) Distribution of equid herpesvirus-1 (EHV-1) in the respiratory tract of ponies: implications for vaccination strategies. Equine Vet J 26:466–469
Liebert M, Washington R, Wedemeyer G, Carey TE, Grossman HB (1994) Loss of co-localization of alpha 6 beta 4 integrin and collagen VII in bladder cancer. Am J Pathol 144:787–795
McGettrick HM, Butler LM, Buckley CD, Rainger GE, Nash GB (2012) Tissue stroma as a regulator of leukocyte recruitment in inflammation. J Leukoc Biol 91:385–400
Mercurio AM (1995) Laminin receptors: achieving specificity through cooperation. Trends Cell Biol 5:419–423
Mestres P, Gomez LL, Lopez TN, del Rosario G, Lukas SW, Hartmann U (2014) The basement membrane of the isolated rat colonic mucosa. A light, electron and atomic force microscopy study. Ann Anat 196:108–118
Müller EJ, Williamson L, Kolly C, Suter MM (2008) Outside-in signaling through integrins and cadherins: a central mechanism to control epidermal growth and differentiation? J Invest Dermatol 128:501–516
Ockleford CD, McCracken SA, Rimmington LA, Hubbard ARD, Bright NA, Cockcroft N, Jefferson TB, Waldron E, d’Lacey C (2013) Type VII collagen associated with the basement membrane of amniotic epithelium forms giant anchoring rivets which penetrate a massive lamina reticularis. Placenta 34:727–773
Osawa T, Abe M, Morigami A, Nozaka Y (2000) Distribution of type VII collagen in the epithelial basement membranes of mouse palate, tongue and lip mucosa. Arch Oral Biol 45:419–424
Rippe B, Davies S (2011) Permeability of peritoneal and glomerular capillaries: what are the differences according to pore theory? Perit Dial Int 31:249–258
Rowe RG, Weiss SJ (2008) Breaching the basement membrane: who, when and how? Trends Cell Biol 18:560–574
Rousselle P, Keene DR, Ruggiero F, Champliaud MF, Rest M, Burgeson RE (1997) Laminin 5 binds the NC-1 domain of type VII collagen. J Cell Biol 138:719–728
Sorokin L (2010) The impact of the extracellular matrix on inflammation. Nat Rev Immunol 10:712–723
Steukers L, Vandekerckhove AP, Van den Broeck W, Glorieux S, Nauwynck HJ (2012) Kinetics of BoHV-1 dissemination in an in vitro culture of bovine upper respiratory tract mucosa explants. ILAR J 53:E43–E54
Timpl R (1989) Structure and biological activity of basement membrane proteins. Eur J Biochem 180:487–502
Van der Meulen KM, Vercauteren G, Nauwynck HJ, Pensaert MB (2003) A local epidemic of equine herpesvirus 1-induced neurological disorders in Belgium. Flemish Vet J 72:366–372
Vandekerckhove A, Glorieux S, Gryspeerdt AC, Steukers L, Osterrieder N, Van de Walle GR, Nauwynck HJ (2010) Replication kinetics of neurovirulent versus non-neurovirulent equine herpesvirus type 1 strains in equine nasal mucosa explants. J Gen Virol 91:2019–2028
Vanlaere I, Libert C (2009) Matrix metalloproteinases as drug targets in infections caused by gram-negative bacteria and in septic shock. Clin Microbiol Rev 22:224–239
Villone D, Fritsch A, Koch M, Bruckner-Tuderman L, Hansen U, Bruckner P (2008) Supramolecular interactions in the dermoepidermal junction zone: anchoring fibrilcollagen VII tightly binds to banded collagen fibrils. J Biol Chem 283:24506–24513
Wang X, Huang DY, Huong SM, Huang ES (2005) Integrin aVb3 is a coreceptor for human cytomegalovirus. Nat Med 11:515–521
Yurchenco PD, Amenta PS, Patton BL (2004) Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol 22:521–538
Yurchenco PD (2011) Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol 3:a004911
Yurchenco PD, Patton BL (2009) Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des 15:1277–1294
Acknowledgments
We would like to thank Prof. H. Favoreel for his scientific contribution, helpful suggestions and fruitful discussions. This research was supported by Ghent University (Concerted Research Action 01G01311). Hossein Bannazadeh Baghi and Hans J Nauwynck are members of the BELVIR consortium (IAP, phase VII) sponsored by the Belgian Science Policy Office (BELSPO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Bannazadeh Baghi, H., Nauwynck, H.J. Effect of equine herpesvirus type 1 (EHV-1) infection of nasal mucosa epithelial cells on integrin alpha 6 and on different components of the basement membrane. Arch Virol 161, 103–110 (2016). https://doi.org/10.1007/s00705-015-2643-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-015-2643-4