Abstract
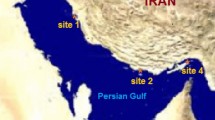
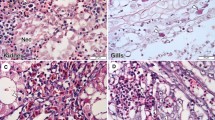
The current epidemiological situation of viral nervous necrosis virus (VNNV) on Hainan Island was investigated. A total of 490 hatchery-reared fish and 652 wild fish were sampled for VNNV detection from March 2013 to May 2014. Positive detection rates of 84.53 % (153/181) and 0.97 % (3/309) were obtained in diseased and healthy hatchery-reared samples, respectively, by conventional RT-PCR. However, using more-sensitive nested RT-PCR, the positive detection rates in healthy hatchery-reared fish reached up to 64.08 % (198/309), suggesting that asymptomatic VNNV carriers commonly exist among larvae and juveniles breeding on Hainan Island. In wild-fish samples, 2.6 % (17/652) and 34.2 % (223/652) positive detection rates were observed using RT-PCR and nested RT-PCR, respectively, indicating that wild fish may be a potential reservoir for VNNV. Phylogenetic analysis showed that all 52 VNNV isolates from cultured fish belong to the RGNNV genotype, but 2 out of 48 VNNV isolates from wild fish samples were found to be of the SJNNV genotype. This study is the first to confirm the existence of SJNNV-genotype VNNV in China. Golden pompano, an important fish species for culture, was selected as a fish model to investigate the optimal conditions for RGNNV disease progression in artificial infection experiments. The effects of temperature, salinity, and fish size were evaluated. Results showed that 28 °C and 20 ‰ are the optimal infection temperature and salinity, respectively, and golden pompano juveniles with small body sizes are more susceptible to RGNNV. These findings are highly consistent with those conditions involved in the natural outbreak of RGNNV.





Similar content being viewed by others
References
Bigarre L, Baud M, Cabon J, Crenn K, Castric J (2010) New PCR probes for detection and genotyping of piscine betanodaviruses. J Fish Dis 33:907–912
Binesh CP, Renuka K, Malaichami N, Greeshma C (2013) First report of viral nervous necrosis-induced mass mortality in hatchery-reared larvae of clownfish, Amphiprion sebae Bleeker. J Fish Dis 36:1017–1020
Chen YM, Wang TY, Chen TY (2014) Immunity to betanodavirus infections of marine fish. Develop Comp Immunol 43:174–183
Chi SC, Shieh JR, Lin SJ (2003) Genetic and antigenic analysis of betanodaviruses isolated from aquatic organisms in Taiwan. Dis Aquat Org 55:221–228
Gjessing MC, Kvellestad A, Ottesen K, Falk K (2009) Nodavirus provokes subclinical encephalitis and retinochoroiditis in adult farmed Atlantic cod, Gadus morhua L. J Fish Dis 32:421–431
Haddad-Boubaker S, Bigarre L, Bouzgarou N, Megdich A, Baud M, Cabon J, Chehida NB (2013) Molecular epidemiology of betanodaviruses isolated from sea bass and sea bream cultured along the Tunisian coasts. Virus Genes 46:412–422
Hick P, Gore K, Whittington R (2013) Molecular epidemiology of betanodavirus-sequence analysis strategies and quasispecies influence outbreak source attribution. Virology 436:15–23
Huang JN, Lin L, Weng SP, He JG (2007) High expression of capsid protein of red-spotted grouper nervous necrosis virus in an avian cell line requires viral RNA2 non-coding regions. J Fish Dis 30:439–444
Iwamoto T, Nakai T, Mori K, Arimoto M, Furusawa I (2000) Cloning of the fish cell line SSN-1 for piscine nodaviruses. Dis Aquat Org 43:81–89
Iwamoto T, Okinaka Y, Mise K, Mori K, Arimoto M, Okuno T, Nakai T (2004) Identification of host-specificity determinants in betanodaviruses by using reassortants between striped jack nervous necrosis virus and sevenband grouper nervous necrosis virus. J Virol 78:1256–1262
Kara HM, Chaoui L, Derbal F, Zaidi R, de Boisseson C, Baud M, Bigarre L (2014) Betanodavirus-associated mortalities of adult wild groupers Epinephelus marginatus (Lowe) and Epinephelus costae (Steindachner) in Algeria. J Fish Dis 37:273–278
Keawcharoen J, Techangamsuwan S, Ponpornpisit A, Lombardini ED, Patchimasiri T, Pirarat N (2015) Genetic characterization of a betanodavirus isolated from a clinical disease outbreak in farm-raised tilapia Oreochromis niloticus (L.) in Thailand. J Fish Dis 38:49–54
Lai YX, Jin BL, Xu Y, Huang LJ, Huang RQ, Zhang Y, Kwang J, He JG, Xie JF (2014) Immune responses of orange-spotted grouper, Epinephelus coioides, against virus-like particles of betanodavirus produced in Escherichia coli. Vet Immunol Immunopathol 157:87–96
Lin L, He J-G, Mori K-I, Nishioka T, Wu J-L, Weng SP, Mushiake K, Arimoto M, Nakai T (2001) Mass mortalities associated with viral nervous necrosis in hatchery-reared groupers in the People’s Republic of China. Fish Pathol 36:186–188
Liu H, Teng Y, Zheng X, Wu Y, Xie X, He J, Ye Y, Wu Z (2012) Complete sequence of a viral nervous necrosis virus (NNV) isolated from red-spotted grouper (Epinephelus akaara) in China. Arch Virol 157:777–782
Liu XD, Huang JN, Weng SP, Hu XQ, Chen WJ, Qin ZD, Dong XX, Liu XL, Zhou Y, Asim M, Wang WM, He JG, Lin L (2015) Infections of nervous necrosis virus in wild and cage-reared marine fish from South China Sea with unexpected wide host ranges. J Fish Dis 38:533–540
Ma HL, Xie JF, Weng SP, Zhou TH, He JG (2012) Co-infection of megalocytivirus and viral nervous necrosis virus in a very severe mass mortality of juvenile orange-spotted groupers (Epinephelus coioides). Aquaculture 358:170–175
Moody NJ, Horwood PF, Reynolds A, Mahony TJ, Anderson IG, Oakey HJ (2009) Phylogenetic analysis of betanodavirus isolates from Australian finfish. Dis Aquat Org 87:151–160
Nishizawa T, Furuhashi M, Nagai T, Nakai T, Muroga K (1997) Genomic classification of fish nodaviruses by molecular phylogenetic analysis of the coat protein gene. Appl Environ Microbiol 63:1633–1636
Olveira JG, Souto S, Dopazo CP, Thiery R, Barja JL, Bandin I (2009) Comparative analysis of both genomic segments of betanodaviruses isolated from epizootic outbreaks in farmed fish species provides evidence for genetic reassortment. J Gen Virol 90:2940–2951
Panzarin V, Fusaro A, Monne I, Cappellozza E, Patarnello P, Bovo G, Capua I, Holmes EC, Cattoli G (2012) Molecular epidemiology and evolutionary dynamics of betanodavirus in southern Europe. Infect Genet Evol 12:63–70
Peng C, Ma H, Su Y, Wen W, Feng J, Guo Z, Qiu L (2015) Susceptibility of farmed juvenile giant grouper Epinephelus lanceolatus to a newly isolated grouper iridovirus (genus Ranavirus). Vet Microbiol 177:270–279
Ransangan J, Manin BO (2012) Genome analysis of Betanodavirus from cultured marine fish species in Malaysia. Vet Microbiol 156:16–44
Shetty M, Maiti B, Shivakumar Santhosh K, Venugopal MN, Karunasagar I (2012) Betanodavirus of marine and freshwater fish: distribution, genomic organization, diagnosis and control measures. Indian J Virol 23:114–123
Su Y, Xu H, Ma H, Feng J, Wen W, Guo Z (2015) Dynamic distribution and tissue tropism of nervous necrosis virus in juvenile pompano (Trachinotus ovatus) during early stages of infection. Aquaculture 440:25–31
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thiery R, Cozien J, de Boisseson C, Kerbart-Boscher S, Nevarez L (2004) Genomic classification of new betanodavirus isolates by phylogenetic analysis of the coat protein gene suggests a low host-fish species specificity. J Gen Virol 85:3079–3087
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Vendramin N, Patarnello P, Toffan A, Panzarin V, Cappellozza E, Tedesco P, Terlizzi A, Terregino C, Cattoli G (2013) Viral encephalopathy and retinopathy in groupers (Epinephelus spp.) in southern Italy: a threat for wild endangered species? BMC Vet Res 9:20
Xu HD, Feng J, Guo ZX, Ou YJ, Wang JY (2010) Detection of red-spotted grouper nervous necrosis virus by loop-mediated isothermal amplification. J Virol Methods 163:123–128
Acknowledgments
The authors would like to thank Prof. Peimao Chen and Peiwen Liang from South China Sea Fisheries Research Institute for identification of wild fishes species. The authors are also grateful to Dr. Junfeng Xie at the School of Life Sciences, Sun Yat-sen University, for kindly providing rabbit anti-VNNV-CP serum. This research was supported by Key Project of Hainan Provincial Science and Technology under number ZDXM20120031, Special Funds of Guangdong Provincial Fiscal Fish Disease Prevention and Control (2012), Science and Technology Planning Project of Guangdong Province (2013B020307008), the Natural Science Foundation of China (31372563), and the Joint Science and Technology Project of SANYA (2011YD09). The nucleotide sequences of threadfin porgy NNV CP genes have been submitted to the NCBI database under GenBank accession number KP994911.
Author information
Authors and Affiliations
Corresponding author
Additional information
H. Ma and W. Wen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ma, H., Wen, W., Su, Y. et al. Epidemiological characterization of VNNV in hatchery-reared and wild marine fish on Hainan Island, China, and experimental infection of golden pompano (Trachinotus ovatus) juveniles. Arch Virol 160, 2979–2989 (2015). https://doi.org/10.1007/s00705-015-2590-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-015-2590-0