Abstract
Human torque teno viruses (TTVs) are new, emerging infectious agents, recently assigned to the family Anelloviridae. The first representative of the genus, torque teno virus (TTV), was discovered in 1997, followed by torque teno mini virus (TTMV) in 2000, and torque teno midi virus (TTMDV) in 2007. These viruses are characterized by an extremely high prevalence, with relatively uniform distribution worldwide and a high level of genomic heterogeneity, as well as an apparent pan-tropism at the host level. Although these viruses have a very high prevalence in the general population across the globe, neither their interaction with their hosts nor their direct involvement in the etiology of specific diseases are fully understood. Since their discovery, human anelloviruses, and especially TTV, have been suggested to be associated with various diseases, such as hepatitis, respiratory diseases, cancer, hematological and autoimmune disorders, with few arguments for their direct involvement. Recent studies have started to reveal interactions between TTVs and the host’s immune system, leading to new hypotheses for potential pathological mechanisms of these viruses. In this review article, we discuss the most important aspects and current status of human TTVs in order to guide future studies.
Similar content being viewed by others
Discovery of torque teno viruses
The torque teno viruses currently known to infect humans include three closely related groups of strains. They were discovered starting in 1997 and have recently been assigned to three genera in the family Anelloviridae: torque teno virus, genus Alphatorquevirus; torque teno mini virus, genus Betatorquevirus; torque teno midi virus, genus Gammatorquevirus [1]. The family Anelloviridae also includes viruses that infect animals, such as pigs (torque teno sus virus, TTsuV), cats (torque teno felis virus), dogs (torque teno canis virus) and tupaias (torque teno tupaia virus) [76]. Anellovirus sequences are continually being described in animals; the most recently discovered sequences were found in UK rodents [131], Tadarida brasiliensis bats [30], and humans [117, 147].
Ever since the discovery of torque teno virus, new viral isolates have been described and identified at a high rate. A brief history of their discovery is presented in Figure 1.
Structure and genome
Structure
TTVs are unenveloped viruses with a capsid (composed of 12 pentameric capsomers) that has T = 1 icosahedral symmetry (http://viralzone.expasy.org/all_by_species/772.html), and the virion has a buoyant density of 1.31–1.34 g/cm3. Filtration studies have shown that the virion particle size ranges between 30 and 50 nm for TTV [124] and is less than 30 nm for TTMV [173].
TTVs cannot be propagated in vitro due to the lack of compatible cell systems, and therefore there have been few studies in which their structure and function have been investigated in a direct manner. The only paper showing an electron microscopy image of TTV virions was published by Itoh et al. [81]. The virions were from sera and fecal supernatant of HIV-infected individuals. Immunogold electron microscopy revealed that they were bound to human immunoglobulin G. However, up to now, no structural studies (e.g., X-ray crystallography) of anelloviruses have been conducted.
Genome
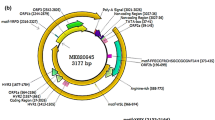
The best-studied component of TTVs, their genome, consists of a circular single-stranded DNA molecule of negative polarity, with a GC-rich region of 117 nucleotides (89 %–90.6 %). Genome size varies between different isolates, ranging from 3.6 to 3.9 kb for TTV [63, 119, 148], 3.24 to 3.25 kb for TTMDV [127], and 2.86 to 2.91 kb for TTMV [142] (Fig. 2).
Schematic illustration of the genome organization of torque teno viruses. a) TTV isolate 2h (GenBank accession no. AY823988), b) TTMDV isolate MD1-073 (GenBank accession no. AB290918), c) TTMV isolate TTMV_LY1 (GenBank accession no. NC_020498.1). The arrows represent open reading frames. Annotations and illustrations were made using UniPro UGENE software version 1.11.4 [145]
The TTV genome can be divided into an untranslated region (UTR) of 1.2 kb and a potential coding region of 2.6 kb. The UTR region is relatively conserved, suggesting that it plays an important regulatory role in viral replication. The coding region of anelloviruses contains two large open reading frames: ORF1, encoding a protein of 770 amino acid residues for TTV (isolate TA278 sequence), 637 amino acid residues for TTMDV (isolate MD1-073 sequence), and 663 amino acid residues for TTMV (isolate CBD231 sequence); and ORF2, encoding of protein of 120, 123 and 91 amino acid residues for TTV (isolate TA278 sequence), TTMDV (isolate MD1-073 sequence) and TTMV (isolate CBD231 sequence). Several other open reading frames have been described, and the peptides that they encode differ in length for different isolates.
TTV isolates are estimated to have 47–70 % divergence in the amino acid sequences of their encoded peptides [11, 99]. These differences are not evenly distributed throughout the genome; most of the variability occurs in the coding region, which has three hypervariable regions [133], while the untranslated regions are less variable than the regulatory sequences [94].
Genome sequence heterogeneity
In the early 2000s, due to the very high genetic variability of these viruses, TTV were classified into five genetic groups with at least 50 % nucleotide sequence divergence. Each group contains a number of genotypes with differences of up to 30 % in terms of genomic sequence [13, 63, 64, 142]. Several different genotypes can co-infect the same individual at the same time, and different combinations of viral genotypes often predominate in different tissues [9].
Phylogenetic analysis has shown that TTVs form a large phylogenetic tree with five major clusters [13]. The reason for the extensive genomic variation and the mechanism by which it occurs is not known, but several hypotheses have been proposed.
One hypothesis is that TTVs mutate at a very high rate. There are many studies in which the nucleotide sequences from multiple isolates were compared, showing either the persistence of the same sequence throughout the years or alterations of the sequence in the host [9, 11, 48, 78].
The high mutation rate is uncharacteristic of DNA viruses, because they lack their own replicative machinery and therefore use the host’s DNA polymerases, which have a high level of proofreading ability. However, it has been shown that the ssDNA genomes of parvoviruses evolve at high rates, and it has been suggested that this increased rate of mutation could be explained by the nature of the single-stranded genome and its capacity to encode proteins involved in replication [167]. It is thus possible for TTV to have a mechanism that allows it to accumulate mutations at a high rate.
The majority of amino acid substitutions in TTV isolates were located in sequences encoded by hypervariable regions of ORF1. The hypervariable regions of the TTV genome may represent mechanisms of immune response avoidance and chronic infection [83].
Another hypothesis suggests that only a small part of TTVs have all the necessary components to successfully infect hosts, or, in other cases, co-infections with multiple isolates may bring together the components needed for infection [91, 99]. Furthermore, a very recent study has demonstrated that a herpesvirus, Epstein-Barr virus, acts as a helper for TTV [17].
Another mechanism by which viruses evolve is recombination, and for TTV, recombination events have been estimated to occur frequently [111, 194]. However, Jelcic et al. [83] have disputed this, arguing that the recombination events that have been reported are artifacts resulting from PCR.
More recently, the intra-genomic recombination has been suggested as a cause of the heterogeneity of the TTV genome [95]. Additionally, frequent recombination phenomena may mask the differences between viral populations [111]. Regarding TTV, it has been suggested that the virus co-evolved with the host over millions of years, resulting in enormous variability [9, 11, 48, 64, 156]. Nonetheless, whatever the mechanism behind TTV heterogeneity, it has become clear that there are no completely monotypic infections, but rather infections with a broad group of viruses [87].
Genome replication
TTV has a high in vivo replication capacity. The kinetics of viral load and clearance during interferon therapy have suggested that more than 1010 TTV virions are produced every day, while 90 % of the virus in the plasma is removed and replaced during this period [103].
TTV genome replication seems to be controlled by the immune system (higher viral titers were found in individuals with immunodeficiencies, such as AIDS [168, 183] and other intercurrent diseases [201]).
Concerning the tropism and cell-specificity of TTV, studies have shown that TTV does not have a particular tropism, and viral genome replication can occur in several tissues/organs (e.g., liver [141], bone marrow [92], lymph nodes, spleen, pancreas, thyroid, muscles, lungs, kidneys, and PMBCs [102, 138, 143, 154]). Nevertheless, viral loads were up to 10–300 times higher in certain tissues (e.g., bone marrow, lung, spleen, liver) than in the serum of the same individual [137]. It was also observed that the TTV genotypes found in plasma and PMBCs often differ [138], which could represent a compartmentalization of the TTV infection similar to that observed with other viruses (e.g., HIV-1, HCV) [100].
The exact mechanism of TTV replication is not fully understood. Based on similarities to other ssDNA-genome viruses, it is assumed that TTV uses the rolling-circle replication mechanism [124]. ORF1 has been shown to encode a protein that contains Rep motifs [43, 99, 124, 181].
The genomic diversity of TTV has also led to the hypothesis that viral DNA is replicated by a machinery with poor or absent proofreading activity. Another hypothesis is that TTV might replicate through an RNA intermediate, like hepatitis B virus, although it seems unlikely because the TTV genome does not encode a reverse transcriptase [133].
Phylogeny
Until recently, TTV formed five major phylogenetic clusters, including tens of viruses with over 35 % nucleotide sequence divergence in ORF1 [16]. Genogroup 3 is believed to contain the largest number of TTV isolates described to date, while genogroups 2 and 4 are less prevalent than the others [14, 41, 60, 104, 151].
Likewise, TTMV is highly divergent; the first phylogenetic tree based on TTMV genomic sequences revealed a large cluster of strains that diverge from each other by over 42 % in ORF1 at the nucleotide level and over 67 % at the amino acid level [15, 173].
Data about TTMDV are scarce; however, recent databases inquiries have shown several complete genomic sequences.
Currently, ICTV classification is based on ORF1 analysis in its entirety, with a cutoff value of 35 % nucleotide sequence identity being the species demarcation criterion for the three anelloviral genera (Alphatorquevirus, Betatorquevirus and Gammatorquevirus). Alphatorquevirus includes 29 species (Torque teno virus 1–29), while Betatorquevirus, which includes 12 species (Torque teno mini virus 1–12), and Gammatorquevirus, which includes 15 species (Torque teno midi virus 1–15), include other related viruses that may be members of these genera but have not been assigned to separate species [1].
To better illustrate the diversity of human anelloviruses, we selected complete ORF1 sequences from GenBank and proceeded to construct the phylogenetic tree (Fig. 3). The tree was built using MEGA 6 software with the UPGMA algorithm, the Jukes-Cantor substitution model and 1000 bootstrap replicates [177]. Tree annotation was performed using EvolView [197]. The name of the isolates used for the phylogenetic analysis along with the accession number and references are found in Online Resource 1.
Phylogenetic tree of human anelloviruses. The tree was built with MEGA 6 software [177], the UPGMA algorithm, the Jukes-Cantor substitution model and 1000 bootstrap replicates and annotated with EvolView [197]. Available complete ORF1 sequences of TTV, TTMV and TTMDV were downloaded from GenBank (Online Resource 1). The reference isolate for each viral species is indicated by a circle
Prevalence of torque teno viruses
TTV, TTMV and TTMDV prevalence in serum and/or blood
The reported prevalence of TTV in blood has varied greatly in recent decades, both within and between different populations around the globe.
Initially it was estimated that the prevalence of TTV is low. Subsequently, information about the heterogeneity of the TTV genome arose, and various PCR methods using different combinations of primers that can detect, quantify and/or differentiate TTV genotypes and related viruses were developed [12, 41, 69, 106, 120, 129].
The rate of TTV-positive sera reported for the general population in the last 5 years varies between 5 % in Brazil [34] and greater than 90 % in Russia, Japan and Pakistan [73, 129, 191]. Despite the fact that the detection rates fluctuate greatly, it is estimated that over 50 % of the general population was infected with TTV in the early 2000s [48, 59, 67].
The highest TTV prevalence has been found in poly-transfused thalassemic patients, long-term hemodialysis patients, hemophiliacs treated with clotting factor, and intravenous drug users. The high TTV titer may reflect the immune status of the individual; immunodepressed patients have high viral DNA titers [183]. Furthermore, monitoring TTV titers in patients undergoing hematopoietic stem cell transplantation has been used to evaluate immune recovery [45, 185].
The shift in the prevalence values reported in the literature over time was due to a large extent to the methods used for the detection of the viral genome. After the discovery of TTV, its detection was done by PCR with primers targeting ORF1 (N22 region, the first described sequence). As UTR regions of the viral genome are more conserved when compared to the ORF regions, UTR-targeting primers (used later for the detection of TTV DNA) detect a larger number of genotypes [65] and thus have a higher overall detection rate.
Regardless of the shortcomings of various techniques, it has become clearer that TTV has a high prevalence in the general population worldwide (Online Resource 2) and that prevalence increases with age [67, 162, 187, 199]. Figure 4 shows a comparative view of TTV prevalence in humans when assessed by amplifying the non-coding vs. the coding region of the TTV genome.
It has been proposed that TTV infection has a different geographical distribution and that some genotypes emerged in isolated ethnic groups [156, 188, 190]; however, several studies have disputed these hypotheses [49, 71, 111, 124, 152].
Studies from the mid 2000s showed that some viral genogroups are more common than others [107, 109], although this may be attributed to the use of certain genogroup-specific primers or even to some peculiarities of the viral genotypes. Moreover, prevalence and viral titer variations can be explained by the use of slightly suboptimal PCR conditions [71].
The other recently discovered human anelloviruses (TTMV and TTMDV) have been studied less, and reports on their prevalence are fewer. The prevalence of TTMV ranges from 48 % in Norway [121] to 80.15 % in Japan [129], whereas TTMDV is present in at least 40 % of the general population [127].
The new detection methods that have been developed are leading to the discovery of new viral variants [15, 126]. The real situation of TTVs prevalence in humans is an important topic for future studies. However, it is clear that the majority of healthy individuals have the virus present in their blood.
TTV prevalence in other biological samples
Using PCR-based methods, TTV DNA has been detected in different organs, tissues and biological samples, including saliva [38, 49, 75, 115, 161], urine [115, 161], perspiration [115], tears [115], nasal secretion [106], feces [49, 115, 136, 161], throat swabs [79], liver [84, 97, 153, 159], bile [186], gastric tissue [105], cervical secretion [23, 46], semen [75, 113, 115], hair and skin [146], bone marrow [141], lymph nodes, muscles, umbilical cord blood [116], thyroid, lungs, spleen, pancreas [141], kidneys, cerebrospinal fluid [101, 153, 169], nervous tissue [169] and PMBCs [143, 196].
In blood, TTV does not have specificity for a given cell type and has been identified in B and T lymphocytes, monocytes, granulocytes and NK cells, but not in red blood cells or platelets [102, 175]. However, there are conflicting views about the presence of viral DNA in B [51] and T lymphocytes [196].
In addition to TTV DNA, its mRNA has been identified in PMBCs, cerebrospinal fluid, breast tissue, colon, bladder, liver, bone marrow and thyroid [112, 139, 153].
TTV DNA has also been detected in tumor tissues [36], including brain tumors [169]. Nonetheless, as noted by Takahashi et al. [175], since TTV is present in blood and immune cells, it is difficult to accurately determine whether the viral DNA detected was derived from the actual tissue cells analyzed or immune cells from the blood flow.
Prevalence of TT viruses in non-biological sources
Given its global ubiquity, fecal-oral route of transmission, lack of seasonal variation, and structural similarity (size and composition) to enteric viruses, TTV is used as an indicator of viral contamination [57].
In Manaus County of the Brazilian Amazon, more than 90 % of the 1.7 million inhabitants are deprived of wastewater collection systems, and thus waste is spilled into the Negro River. A team of researchers collected 52 water samples four times (August, November, February and June) from 13 locations in the region for a period of one year. TTV was detected in 92.3 % of the samples of surface water, ranging from 1,300 to 746,000 TTV genomes in 100 ml of water. The presence of TTV DNA did not fluctuate according to season and geographical area, and the TTV load did not correlate with the abundance of coliform bacteria or physicochemical parameters [42]. Nevertheless, the prevalence of TTV was similar to the prevalence of HCV reported in 2007 in the same geographical area [35].
In Europe, to assess the rate of TTV positivity in Italian waters, Veran et al. collected samples of treated river water monthly for one year reporting that TTV was present in 3 out of 12 samples (25 %) [192].
Studies in Japan showed that TTV DNA was present in 5 % of the surface water in that country, without seasonal variation [61]. When TTV was monitored in eight active sludge-treatment plants in Japan every month for 1 year, researchers reported that TTV genetic material was detected with a frequency of 97 % in the influent and 18 % in secondary effluent after treatment with activated sludge before chlorination, 24 % in the final effluent after chlorination and 0 % in the effluent for reuse after filtration and ozonation [62]. They also concluded that chlorination does not affect PCR detection of TTV genetic material, although it can inactivate the virus without affecting the genomic regions amplified [134].
In India, Vaidya et al. [189] compared the titers of hepatitis A and E viruses and TTV in influents and effluents of wastewater treatment plants. They observed that the prevalence of TTV DNA in untreated wastewater (12.7 %) was statistically similar to the prevalence of hepatitis E virus RNA (11.0 %) and hepatitis A virus RNA (24.4 %). In effluent water, unlike hepatitis A virus, TTV and hepatitis E virus did not show statistically significant decreases.
TTV DNA was also detected on 14.9 % of hospital surfaces tested by Carducci et al. [21] and on 31 % of surfaces, as well as 16 % of air samples contaminated through toilet use [193]. The authors suggested that TTV could be used as an indicator of general viral contamination of the environment.
Transmission routes
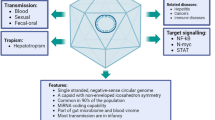
The main route of transmission of TTVs is believed to be by blood, as it is commonly present in people exposed to infected blood and blood products (e.g., transfusions or hemodialysis) [2, 77, 114, 157], or in people using intravenous drugs [6, 7]. Even so, parenteral transmission (e.g., by injection) does not explain the global prevalence and ubiquity of TTV viruses. Viruses are transmitted efficiently through saliva, and TTV is no exception; its titer in human saliva may exceed that in serum [38, 49]. The presence of TTV DNA in pharyngeal mucus [29] suggests that respiratory tract and salivary droplets are common dissemination paths. Moreover, a recent study has provided evidence of the presence of TTV in the breath of infected individuals [27]. It is possible that the main route of transmission would involve breath and saliva, as this would explain its high prevalence.
In infected individuals, TTV is also present in feces [98, 136], and TTV isolated from this source is able to infect susceptible and permissive cells in vitro [101]. Fecal-oral transmission of TTV is most likely achieved through progeny virions secreted in the bile, which are released later in feces [136, 186].
A secondary enteric transmission route of TTVs is by water. TTV has been identified in a number of aquatic ecosystems, being detected in 5 % of the surface water tested in Japan [61], but mostly in sewage water [62]. Griffin et al. [57] have proposed that international standards of water quality testing should include detection and quantification of TTV; current tests are based only on bacterial indicators, most often providing erroneous information on the presence of enteric viruses. Thus, TTV can become an effective indicator for risk assessment for viral pathogens in water.
TT viruses have recently been detected in food, supporting the oral route of transmission. TTV DNA was found in raw and pasteurized camel milk from the United Arab Emirates [3] and in raw, but not pasteurized, buffalo milk and dairy products from Italy [160]. TTV and TTMV DNA were also detected in meat and meat products collected from local supermarkets in Romania [170].
Given the high rates of infection with TTVs, alternative routes of transmission have been considered, including transplacental transmission, transmission through the umbilical cord blood [123, 162], or contact with saliva, skin, or hair of infected individuals [146].
Sexual transmission is another possible route of transmission of TTVs in the human adult population, considering that they have been detected in the cervical epithelium and in semen [19, 23, 44, 75, 113]; recent studies have shown that TTV has the ability to multiply with high frequency in the cervical epithelium [198]. However, some researchers believe that sexual transmission is of minor importance for TTV given the fact that the infection may occur early in life or even in utero, and various TTV genotypes have been detected in newborns, umbilical cord blood and amniotic fluid [8, 55, 116, 123, 162]. Other findings contradict the hypothesis of intrauterine transmission of TTV, claiming that healthy newborns may come in contact with the virus a few months after birth from the environment [33, 90, 129] or breast milk [55, 80, 116, 135, 165]. The conflicting data obtained may be the consequence of the PCR methods used for viral DNA detection [116] and the low viral load in children.
TTV sequence comparison from isolates identified in children and their mothers have shown both similarity and divergence, confirming that TTV is acquired by children in more than one way – from the mother and from the environment [8, 33, 96, 135, 171].
As for the newly discovered TTVs, TTMV has been detected in umbilical cord blood, breast milk, amniotic fluid, and serum of newborns, and these viruses show sequence similarity to those of the mother [116]. The mother-child transmission route may explain the apparently ubiquitous and persistent infections.
Involvement in human pathology
TTVs are characterized by a high prevalence in the general population. The possible involvement of these viruses in human pathologies has been debated ever since their discovery. Certainly, a nearly ubiquitous virus is unlikely to be pathogenic per se. Although TTV, TTMDV and TTMV are potentially associated with many diseases, there are conflicting opinions about their disease-causing potential in the human populations due to their ubiquity. It is possible that only certain genotypes/genogroups/species of human anelloviruses may be particularly pathogenic.
While TTV was originally believed to be a new viral etiologic agent of hepatitis [132], following the description of the virus’ prevalence in most of the general population of the globe, researchers have tried to associate it with a specific or non-specific pathology. In 1999, Griffiths et al. [58] introduced the concept of a commensal virus to describe the TTV-host relationships.
Those who are convinced of the lack of TTV pathogenicity believe that this could be the result of long periods of virus-host co-evolution or the lack of specific cell ligands. Nevertheless, epidemiological studies have provided evidence about the association of TTV with several pathological conditions of the liver, blood, immune system, respiratory system, cancers, etc. However, these studies do not paint an accurate and complete picture of the involvement of TTV in human pathology [54].
In the last decade, only a few studies have started to scratch the surface of the presumable pathogenic potential of TTV and the mechanisms behind it. The expression of genotype 1 TTV ORF1 in transgenic mice produces a protein that causes pathological changes in the kidney. This protein appears to interfere with renal epithelial cell differentiation [195]. A recent study has shown that certain TTV isolates are activators of pro-inflammatory cytokine production through Toll-like receptor 9 (TLR-9) and thus may increase the severity of inflammatory diseases [158]. Moreover, Kincaid et al. [93] showed that TTV encodes an miRNA in vivo that targets N-myc (and STAT) interactor (NMI), thus mediating a decreased response to IFN and increased cellular proliferation in the presence of IFN. These facts support the theory that miRNA-mediated immune evasion contributes to the immense ubiquity of these viruses.
The pathogenic mechanisms of TTVs remain cryptic and still constitute the tabula rasa of TTVs research.
Hepatitis
TTV has been discovered in a case of post-transfusion hepatitis. Furthermore, it was shown that it can multiply in hepatocytes both in vivo [140] and in vitro [39], and therefore numerous subsequent studies were focused on the involvement of TTV in liver pathology.
Okamoto et al. reported TTV titers 10 to 100-fold higher in liver tissue than in serum from patients with transfusion-acquired hepatitis [137]. They also noted that in recently infected patients, alanine transaminase (ALT) levels increased with viral titer, with TTV becoming undetectable in patients with normalized ALT levels [132].
Given the high rate of asymptomatic infection with TTV in the general population, it is most likely that hepatitis is not caused by TTV infection per se [5, 130]. However, TTV has been associated with fulminant hepatitis [24, 25, 72, 125]. Tanaka [179] and Desai et al. [40] reported that abnormal liver profiles were common among viremic individuals. A previous study that focused on highlighting the cytopathic effects of TTV in hepatocytes showed that liver function in most cases of hepatitis with TTV is abnormal; liver tissue appears discolored, cells are swollen, and acidophil degeneration, formation of apoptotic bodies and focused necrosis develop. However, inflammation of the lobules and portal system is mild [70].
Another series of studies reported possible associations between TTV infection and liver pathology [24, 74, 88, 144, 178, 184] such as recurrent acute hepatitis (genotype 13 TTV), reinforcing the hypothesis that TTV may be involved in mild forms of liver disease [47]. It has also been suggested that persistent TTV infections can contribute to cryptogenic liver failure in hemophiliacs [176].
A further assumption made about the involvement of TTV in liver pathology is that certain viral genotypes have a higher pathogenic potential than others, or even that some particular genotypes are associated with hepatitis. Genotype 1 has been incriminated in the occurrence of post-transfusion hepatitis [180], and genotype 1a has been reported predominantly in children with fulminant hepatitis and chronic hepatitis [144].
Clinical significance of co-infections with TTV and hepatitis viruses
Concurrent infections with TTV and hepatitis viruses (HBV and HCV) have been extensively studied. High TTV viral loads have been associated with the development of hepatocellular carcinoma in patients with chronic hepatitis C [182]; moreover, the histological grade appears to be higher in hepatocellular patients with TTV-HCV co-infection than in those infected with only HCV [164]. Moreover, it has been suggested that ORF3 of TTV genotype 1a encodes a protein similar to the non-structural protein 5A (NS5A) encoded by HCV. This protein plays a major role in the IFN-induced antiviral response [182].
The mortality rate in patients with HBV-TTV co-infection is higher than in patients with HBV infection alone [40]. However, no clear evidence has been provided for the involvement of TTV as a causative agent, co-factor or marker for the occurrence or progression of liver disease.
Another series of studies has shown that suprainfection of HBV or HCV patients with TTV does not seem to have any effect on the severity of disease manifestations [20, 68, 89, 166, 184]. Although TTV is frequently detected in patients chronically infected with HBV and HCV, it does not seem to have any influence on the histopathological characteristics of the HBV- or HCV-infected liver. Chattopadhyay et al. have shown that the clinical and biochemical profiles of patients with chronic hepatitis or HBV and HCV co-infection with TTV were not significantly different from those without TTV [26]. However, the possibility that TTV contributes to the progression of liver disease in people infected with HBV or HCV cannot be totally excluded [40, 122].
Data on the relationship between TTV and liver diseases have been found to be inconsistent, since there is an extensive range of evidence indicating the absence of any such correlation. The lack of morphological changes in hepatocytes that are positive for TTV DNA by in situ hybridization casts doubt on a role for TTV in liver pathogenesis.
Kadayifici et al. [86] compared the rate of detection of viral DNA in patients with elevated ALT and healthy people and did not find any significant difference. Histological examination also did not reveal any visible cytopathic effect that could be attributed to viral infection with TTV.
Once again, the conflicting data leave the matter of TTV involvement in liver diseases unresolved.
Respiratory diseases
Lung tissue has been shown to be one of the sites where TTV can multiply [10]. TTV infection has been suggested to play a role (either as an active or opportunistic pathogen) in children with acute respiratory disease. Higher TTV viral loads were found in nasal swabs from patients with severe respiratory diseases such as bronchopneumonia [106]. Also, TTMDV prevalence appears to be higher in children with acute respiratory distress syndrome [29]. Furthermore, children with increased viral titer in nasal specimens were found to have weak spirometric values. It also appears that TTV may contribute to the pathogenesis of asthma; it has been hypothesized that TTV replication could direct the immune response toward the Th2 pathway, which is known to be involved in asthma pathogenesis [149].
In patients with idiopathic pulmonary fibrosis, TTV infections are associated with the severity of bronchiectasis, and even with a low rate of survival [150]. Also, a TTMV isolate, namely TTMV-LY, was identified in pleural effusion and may potentially be associated with severe pneumonia in children. The authors found that TTMV-LY can deeply colonize the lungs; alveolar epithelial cells are permissive for TTMV-LY. The infection modulates the innate immune response in lung cells by inducing the expression of pro-inflammatory cytokines (IFN-γ, CCL5, IL-2 and IL-12) [50].
However, as in the case of the other diseases mentioned above, it is not clear whether TTV infections are the cause or the result of disease progression.
Cancer
Consistent with the ubiquity of human anelloviruses, TTV DNA has been found in a variety of neoplastic tissues [36]. However, this could be supported by tissue inflammation or rapid division of cancer cells that support the replication of the TTV genome.
zur Hausen et al. have put forward the interesting hypothesis that persistent infections with TTV increase the risk of specific translocations, thus having an effect on the development of leukemia and lymphoma in children [202]. Moreover, TTV genotype 10 was identified in a patient with chronic lymphocytic leukemia and polycythemia vera [28]. Although TTV DNA was identified in various types of lymphoma with the same prevalence as in healthy individuals and the virus was found in non-neoplastic cells, some researchers have postulated that TTV may modulate infected T lymphocytes and thus play a role in the pathogenesis of lymphomas [51].
Co-infection with human papillomavirus (HPV) and TTV genotype 1 has been associated with poor prognosis in patients with laryngeal cancer, but it is unknown whether TTV has any effect on cancer progression [172].
TTV DNA has been detected with a significantly higher frequency in patients with sporadic breast carcinoma in association with the eNOS bb (eNOS 4a/5b VNTR) genotype or the presence of the ACE I (ACE ID VNTR) allele compared to healthy women [31].
The presence of TTV in colorectal carcinoma specimens may be associated with pathogenesis, but the same TTV isolates have also been detected in normal tissue [37].
TTV DNA is frequently detected in sera from patients with Kaposi’s sarcoma. Furthermore, TTV DNA is detected in the epithelial cells of the lesions and normal skin of these patients, but not from healthy subjects. It has been suggested that TTV infection might have an effect on the replication of the human herpesvirus 8, and thus a role in the pathogenesis of the disease [56].
TTV has been identified at a tenfold higher rate in PMBCs of patients with various types of cancer than in healthy subjects. However, it is unclear whether this is a feature of neoplastic pathology or it if is common in patients with severe diseases in general [200].
Hematological disorders
Hepatitis-associated aplastic anemia occurs in particular after non-A, non-B, non-C acute hepatitis [18]. High levels of TTV in the bone marrow have been suggested to be responsible for aplastic anemia of unknown etiology [92, 118]. This possible association, however, was not supported in other studies [155, 163].
TTV has been found with high prevalence, ranging between 50.5 % [4] and 100 % [22], in patients with thalassemia. Studies have also shown that thalassemic patients were positive for TTV DNA in different biological samples (e.g., PMBC, plasma, saliva, urine) with the highest rate of prevalence in PMBCs and plasma (greater than 90 %), and the lowest prevalence in urine (22 %). Also, more than 45 % of the patients had co-infections, and the most frequently found genogroup of TTV was genogroup 2 [22].
Regarding the association of TTV with hematopoietic malignancies, TTV DNA was detected in lymphocytes and lymph nodes from patients with B-cell and Hodgkin’s lymphoma [51]. It has been postulated that TTV may modulate the functions of infected T cells and thus may play a role in the pathogenesis of lymphoma.
Autoimmune disorders
The etiological agents for many autoimmune diseases are not yet known, and investigations have been carried out, taking into consideration the subtle interplay between TTV and the immune system. Costa et al. reported the presence of TTV and even co-infection with multiple TTV genotypes in patients with systemic lupus erythematosus (SLE). Although this study revealed an association between TTV infection and SLE, the number of TTV genotypes infecting the patients did not appear to have an effect on SLE phenotype [32].
Gergely et al. observed that peptides encoded by TTV ORF1 and ORF2 show similarity to a human endogenous retrovirus-encoded nuclear protein (HRES-1/p28), which generates antibody epitopes common in cases of SLE. In addition to the high titers of TTV, patients also had high titers of anti-ORF1 and anti-ORF2 antibodies. However, it is unclear whether TTV plays a role in the production of these auto-antibodies, or if, due to immunological causes, patients may be more prone to infections [53].
TTV has been found in patients with rheumatoid arthritis [53, 66], idiopathic inflammatory myopathy [52], and multiple sclerosis (MS) [169]. Sospedra et al. have shown that T cells derived from MS patients reacted against arginine-rich peptide regions that were similar to the amino termini of TTMV and TTV ORF1 peptides. They hypothesized that recurrent infection with TTV and other predisposing factors (genetic and/or environmental) lead to the expansion of these cells and have an impact on disease development [169].
Maggi et al. showed that patients with rheumatoid arthritis had higher titers of TTV than healthy subjects. The authors suggest that there may be a link between TTV and arthritis, but, as in the case of other diseases, a direct relationship remains in question [110].
A study using an animal model showed that the expression of TTV genotype 1 ORF1 in transgenic mice leads to a truncated protein that causes pathological changes in kidney cells. The expression of the protein appears to interfere with the differentiation of kidney epithelial cells [195].
Although TTV is potentially associated with several human diseases, its presence in the general population across the globe, the uncertainty of a virus-free negative control, and the lack of obvious cytopathic and cytotoxic effects on cells make opinions related to the pathogenicity of these viruses controversial and difficult to sustain. It has been suggested that small TTV sub-genomic fragments identified in human serum could play a role in diseases, as is the case with plant geminiviruses [95]. It is also possible that some genotypes/genogroups could be more pathogenic than others [106, 110], as in the case with HPV.
On the other hand, until it is definitively demonstrated that TTVs have a pathogenic nature, it must be taken into account that TTVs replicate in PMBCs and are inevitably found in infected or damaged tissues. Thus, the presence or load variation of TTV DNA in tissue samples cannot constitute a direct association between TTV and certain pathology. Thus, it is possible that the higher TTV titers observed in different patient groups are a secondary phenomenon due to the disease [14].
The pathogenic potential of TTVs is still unclear, although there are numerous studies that have investigated the relationship between them and different pathologies (e.g., liver disease0, acute respiratory disorders, AIDS development, various types of cancer, autoimmune disorders and kidney disease). TTV replication appears to be stimulated by vaccination with an antigen, suggesting that the viral titer may be enhanced due to co-infection with other pathogens [108]. TTV infection with multiple genotypes and low and fluctuating viremia are making the discovery of association with different pathologies extremely difficult. New PCR systems that enable the detection, differentiation and quantification of all genotypes and/or genogroups are desired.
Conclusions
As TTV and its relatives appear to have infected primates and probably all mammals throughout their evolutionary history, an intriguing question is whether the absence of TTV infection would have any consequences, such as immune system dysregulation that may result from the absence of the substantial antigenic burden of TTVs in infected individuals. For all we know now, TTV-related viruses may have been key players in the vast process of evolution. These unresolved questions undoubtedly represent fertile areas for fundamental virology and immunology research in the future.
References
(2012) Family—Anelloviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (eds) Virus taxonomy. Elsevier, San Diego, pp 331–341
Afkari R, Pirouzi A, Mohsenzadeh M, Azadi M, Jafari M (2012) Molecular detection of TT virus and SEN virus infections in hemodialysed patients and blood donors in south of Iran. Indian J Pathol Microbiol 55:478–480
Al-Moslih MI, Perkins H, Hu YW (2007) Genetic relationship of Torque Teno virus (TTV) between humans and camels in United Arab Emirates (UAE). J Med Virol 79:188–191
Alavi S, Sharifi Z, Valeshabad AK, Nourbakhsh K, Shamsian BS, Arzanian MT, Safarisharari A, Navidinia M (2011) Clinical outcomes of Torque teno virus-infected thalassemic patients with and without hepatitis C virus infection. Korean J Hematol 46:123–127
Allain JP (2000) Emerging viral infections relevant to transfusion medicine. Blood Rev 14:173–181
Alzahrani AJ, Dela Cruz DM, Obeid OE, Bukhari HA, Al-Qahtani AA, Al-Ahdal MN (2009) Molecular detection of hepatitis B, hepatitis C, and torque teno viruses in drug users in Saudi Arabia. J Med Virol 81:1343–1347
Ataei B, Emami Naeini A, Khorvash F, Yazdani MR, Javadi AA (2012) Prevalence of transfusion transmitted virus infection in hemodialysis patients and injection drug users compared to healthy blood donors in Isfahan, Iran. Gastroenterol Res Pract 2012:671927
Bagaglio S, Sitia G, Prati D, Cella D, Hasson H, Novati R, Lazzarin A, Morsica G (2002) Mother-to-child transmission of TT virus: sequence analysis of non-coding region of TT virus in infected mother-infant pairs. Arch Virol 147:803–812
Ball JK, Curran R, Berridge S, Grabowska AM, Jameson CL, Thomson BJ, Irving WL, Sharp PM (1999) TT virus sequence heterogeneity in vivo: evidence for co-infection with multiple genetic types. J Gen Virol 80(Pt 7):1759–1768
Bando M, Ohno S, Oshikawa K, Takahashi M, Okamoto H, Sugiyama Y (2001) Infection of TT virus in patients with idiopathic pulmonary fibrosis. Respir Med 95:935–942
Biagini P, Gallian P, Attoui H, Cantaloube JF, de Micco P, de Lamballerie X (1999) Determination and phylogenetic analysis of partial sequences from TT virus isolates. J Gen Virol 80(Pt 2):419–424
Biagini P, Gallian P, Attoui H, Cantaloube JF, Touinssi M, de Micco P, de Lamballerie X (2001) Comparison of systems performance for TT virus detection using PCR primer sets located in non-coding and coding regions of the viral genome. J Clin Virol 22:91–99
Biagini P (2004) Human circoviruses. Vet Microbiol 98:95–101
Biagini P, Gallian P, Cantaloube JF, Attoui H, de Micco P, de Lamballerie X (2006) Distribution and genetic analysis of TTV and TTMV major phylogenetic groups in French blood donors. J Med Virol 78:298–304
Biagini P, Uch R, Belhouchet M, Attoui H, Cantaloube JF, Brisbarre N, de Micco P (2007) Circular genomes related to anelloviruses identified in human and animal samples by using a combined rolling-circle amplification/sequence-independent single primer amplification approach. J Gen Virol 88:2696–2701
Biagini P (2009) Classification of TTV and related viruses (anelloviruses). Curr Top Microbiol Immunol 331:21–33
Borkosky SS, Whitley C, Kopp-Schneider A, zur Hausen H, de Villiers EM (2012) Epstein-Barr virus stimulates torque teno virus replication: a possible relationship to multiple sclerosis. PLoS ONE 7:e32160
Brown KE, Wong S, Young NS (1997) Prevalence of GBV-C/HGV, a novel ‘hepatitis’ virus, in patients with aplastic anaemia. Br J Haematol 97:492–496
Calcaterra S, Zaniratti MS, Serraino D, Peroni M, Abbate I, Cappiello G, Piselli P, Pavia C, Rezza G, Ippolito G, Capobianchi MR (2001) Cervicovaginal shedding of TT virus in HIV-infected women. J Hum Virol 4:343–345
Campo N, Torre E, Brizzolara R, Sinelli N, Russo R, Puppo F, Picciotto A (2000) Prevalence of TT virus (TTV) infection in patients at high risk of parenterally transmitted viruses. J Hepatol 32:185
Carducci A, Verani M, Lombardi R, Casini B, Privitera G (2011) Environmental survey to assess viral contamination of air and surfaces in hospital settings. J Hosp Infect 77:242–247
Chan PK, Chik KW, Li CK, Tang NL, Ming MS, Cheung JL, Ng KC, Yuen PM, Cheng AF (2001) Prevalence and genotype distribution of TT virus in various specimen types from thalassaemic patients. J Viral Hepat 8:304–309
Chan PK, Tam WH, Yeo W, Cheung JL, Zhong S, Cheng AF (2001) High carriage rate of TT virus in the cervices of pregnant women. Clin Infect Dis 32:1376–1377
Charlton M, Adjei P, Poterucha J, Zein N, Moore B, Therneau T, Krom R, Wiesner R (1998) Prevalence of TT-virus infection in North American blood donors, patients with fulminant hepatic failure and cryptogenic cirrhosis. Hepatology 28:263a–263a
Charlton M, Adjei P, Poterucha J, Zein N, Moore B, Therneau T, Krom R, Wiesner R (1998) TT-virus infection in North American blood donors, patients with fulminant hepatic failure, and cryptogenic cirrhosis. Hepatology 28:839–842
Chattopadhyay S, Das BC, Gupta RK, Kar P (2005) Presence of TT virus infection in chronic hepatitis patients from a hospital in New Delhi, India. Indian J Med Res 122:29–33
Chikasue K, Kimura M, Ikeda K, Ohnishi T, Kawanishi S, Iio T, Kataoka M, Arao Y (2012) Detection of Torque teno virus DNA in exhaled breath by polymerase chain reaction. Acta Med Okayama 66:387–397
Chu CC, Zhang L, Dhayalan A, Agagnina BM, Magli AR, Fraher G, Didier S, Johnson LP, Kennedy WJ, Damle RN, Yan XJ, Patten PE, Teichberg S, Koduru P, Kolitz JE, Allen SL, Rai KR, Chiorazzi N (2011) Torque teno virus 10 isolated by genome amplification techniques from a patient with concomitant chronic lymphocytic leukemia and polycythemia vera. Mol Med 17:1338–1348
Chung JY, Han TH, Koo JW, Kim SW, Seo JK, Hwang ES (2007) Small anellovirus infections in Korean children. Emerg Infect Dis 13:791–793
Cibulski SP, Teixeira TF, de Sales Lima FE, do Santos HF, Franco AC, Roehe PM (2014) A novel Anelloviridae species detected in Tadarida brasiliensis bats: first sequence of a chiropteran anellovirus. Genome Announc 2
Cimponeriu D, Ion DA, Spandole S, Apostol P, Toma M, Radu I, Panduru N, Belc N, Berca LM, Adascalului M, Niculae OM, Burcos T, Popa E, Popa I, Stanilescu S, Belusica L, Cristian S (2013) Potential implication of genetic polymorphisms and Torque teno virus in sporadic breast cancer. Rom Biotechnol Lett 18:7889–7896
Costa MR, Costa IP, Devalle S, Castro AR, Freitas SZ (2012) Prevalence and genetic diversity of torque teno virus in patients with systemic lupus erythematosus in a reference service in Mato Grosso do Sul. Rev Bras Reumatol 52:49–54
Davidson F, MacDonald D, Mokili JL, Prescott LE, Graham S, Simmonds P (1999) Early acquisition of TT virus (TTV) in an area endemic for TTV infection. J Infect Dis 179:1070–1076
de Oliveira JC, Nasser TF, Oda JM, Aoki MN, Carneiro JL, Barbosa DS, Reiche EM, Watanabe MA (2008) Detection of TTV in peripheral blood cells from patients with altered ALT and AST levels. New Microbiol 31:195–201
De Paula VS, Diniz-Mendes L, Villar LM, Luz SL, Silva LA, Jesus MS, da Silva NM, Gaspar AM (2007) Hepatitis A virus in environmental water samples from the Amazon Basin. Water Res 41:1169–1176
de Villiers EM, Schmidt R, Delius H, zur Hausen H (2002) Heterogeneity of TT virus related sequences isolated from human tumour biopsy specimens. J Mol Med (Berl) 80:44–50
de Villiers EM, Bulajic M, Nitsch C, Kecmanovic D, Pavlov M, Kopp-Schneider A, Lohr M (2007) TTV infection in colorectal cancer tissues and normal mucosa. Int J Cancer 121:2109–2112
Deng X, Terunuma H, Handema R, Sakamoto M, Kitamura T, Ito M, Akahane Y (2000) Higher prevalence and viral load of TT virus in saliva than in the corresponding serum: another possible transmission route and replication site of TT virus. J Med Virol 62:531–537
Desai M, Pal R, Deshmukh R, Banker D (2005) Replication of TT virus in hepatocyte and leucocyte cell lines. J Med Virol 77:136–143
Desai MM, Pal RB, Banker DD (2005) Molecular epidemiology and clinical implications of TT virus (TTV) infection in Indian subjects. J Clin Gastroenterol 39:422–429
Devalle S, Niel C (2004) Distribution of TT virus genomic groups 1–5 in Brazilian blood donors, HBV carriers, and HIV-1-infected patients. J Med Virol 72:166–173
Diniz-Mendes L, Paula VS, Luz SL, Niel C (2008) High prevalence of human Torque teno virus in streams crossing the city of Manaus, Brazilian Amazon. J Appl Microbiol 105:51–58
Erker JC, Leary TP, Desai SM, Chalmers ML, Mushahwar IK (1999) Analyses of TT virus full-length genomic sequences. J Gen Virol 80(Pt 7):1743–1750
Fabrizi F, Martin P, Lunghi G, Locatelli F (2001) TT virus infection in end-stage renal disease (ESRD). J Nephrol 14:80–87
Focosi D, Maggi F, Albani M, Macera L, Ricci V, Gragnani S, Di Beo S, Ghimenti M, Antonelli G, Bendinelli M, Pistello M, Ceccherini-Nelli L, Petrini M (2010) Torquetenovirus viremia kinetics after autologous stem cell transplantation are predictable and may serve as a surrogate marker of functional immune reconstitution. J Clin Virol 47:189–192
Fornai C, Maggi F, Vatteroni ML, Pistello M, Bendinelli M (2001) High prevalence of TT virus (TTV) and TTV-like minivirus in cervical swabs. J Clin Microbiol 39:2022–2024
Foschini MP, Morandi L, Macchia S, DalMonte PR, Pession A (2001) TT virus-related acute recurrent hepatitis. Histological features of a case and review of the literature. Virchows Arch 439:752–755
Gallian P, Berland Y, Olmer M, Raccah D, de Micco P, Biagini P, Simon S, Bouchouareb D, Mourey C, Roubicek C, Touinssi M, Cantaloube JF, Dussol B, de Lamballerie X (1999) TT virus infection in French hemodialysis patients: study of prevalence and risk factors. J Clin Microbiol 37:2538–2542
Gallian P, Biagini P, Zhong S, Touinssi M, Yeo W, Cantaloube JF, Attoui H, de Micco P, Johnson PJ, de Lamballerie X (2000) TT virus: a study of molecular epidemiology and transmission of genotypes 1, 2 and 3. J Clin Virol 17:43–49
Galmes J, Li Y, Rajoharison A, Ren L, Dollet S, Richard N, Vernet G, Javouhey E, Wang J, Telles JN, Paranhos-Baccala G (2012) Potential implication of new torque teno mini viruses in parapneumonic empyema in children. Eur Respir J 42:470
Garbuglia AR, Iezzi T, Capobianchi MR, Pignoloni P, Pulsoni A, Sourdis J, Pescarmona E, Vitolo D, Mandelli F (2003) Detection of TT virus in lymph node biopsies of B-cell lymphoma and Hodgkin’s disease, and its association with EBV infection. Int J Immunopathol Pharmacol 16:109–118
Gergely P Jr, Blazsek A, Danko K, Ponyi A, Poor G (2005) Detection of TT virus in patients with idiopathic inflammatory myopathies. Ann N Y Acad Sci 1050:304–313
Gergely P Jr, Pullmann R, Stancato C, Otvos L Jr, Koncz A, Blazsek A, Poor G, Brown KE, Phillips PE, Perl A (2005) Increased prevalence of transfusion-transmitted virus and cross-reactivity with immunodominant epitopes of the HRES-1/p28 endogenous retroviral autoantigen in patients with systemic lupus erythematosus. Clin Immunol 116:124–134
Gergely P Jr, Perl A, Poor G (2006) Possible pathogenic nature of the recently discovered TT virus: does it play a role in autoimmune rheumatic diseases? Autoimmun Rev 6:5–9
Gerner P, Oettinger R, Gerner W, Falbrede J, Wirth S (2000) Mother-to-infant transmission of TT virus: prevalence, extent and mechanism of vertical transmission. Pediatr Infect Dis J 19:1074–1077
Girard C, Ottomani L, Ducos J, Dereure O, Carles MJ, Guillot B (2007) High Prevalence of Torque teno (TT) virus in classical Kaposi’s sarcoma. Acta Derm Venereol 87:14–17
Griffin JS, Plummer JD, Long SC (2008) Torque teno virus: an improved indicator for viral pathogens in drinking waters. Virol J 5:112
Griffiths P (1999) Time to consider the concept of a commensal virus? Rev Med Virol 9:73–74
Hafez MM, Shaarawy SM, Hassan AA, Salim RF, Abd El Salam FM, Ali AE (2007) Prevalence of transfusion transmitted virus (TTV) genotypes among HCC patients in Qaluobia governorate. Virol J 4:135
Hallett RL, Clewley JP, Bobet F, McKiernan PJ, Teo CG (2000) Characterization of a highly divergent TT virus genome. J Gen Virol 81:2273–2279
Haramoto E, Katayama H, Oguma K, Ohgaki S (2005) Application of cation-coated filter method to detection of noroviruses, enteroviruses, adenoviruses, and torque teno viruses in the Tamagawa River in Japan. Appl Environ Microbiol 71:2403–2411
Haramoto E, Katayama H, Oguma K, Yamashita H, Nakajima E, Ohgaki S (2005) One-year monthly monitoring of Torque teno virus (TTV) in wastewater treatment plants in Japan. Water Res 39:2008–2013
Heller F, Zachoval R, Koelzer A, Nitschko H, Froesner GG (2001) Isolate KAV: a new genotype of the TT-virus family. Biochem Biophys Res Commun 289:937–941
Hijikata M, Takahashi K, Mishiro S (1999) Complete circular DNA genome of a TT virus variant (isolate name SANBAN) and 44 partial ORF2 sequences implicating a great degree of diversity beyond genotypes. Virology 260:17–22
Hino S (2002) TTV, a new human virus with single stranded circular DNA genome. Rev Med Virol 12:151–158
Hirata D, Kaneko N, Iwamoto M, Yoshio T, Okazaki H, Mimori A, Masuyama J, Minota S (1998) Infection with an unenveloped DNA virus (TTV) associated with non-A to G hepatitis in patients with rheumatoid arthritis. Br J Rheumatol 37:1361–1362
Hsieh SY, Wu YH, Ho YP, Tsao KC, Yeh CT, Liaw YF (1999) High prevalence of TT virus infection in healthy children and adults and in patients with liver disease in Taiwan. J Clin Microbiol 37:1829–1831
Hsu HY, Ni YH, Chen HL, Kao JH, Chang MH (2003) TT virus infection in healthy children, children after blood transfusion, and children with non-A to E hepatitis or other liver diseases in Taiwan. J Med Virol 69:66–71
Hu YW, Al-Moslih MI, Al Ali MT, Khameneh SR, Perkins H, Diaz-Mitoma F, Roy JN, Uzicanin S, Brown EG (2005) Molecular detection method for all known genotypes of TT virus (TTV) and TTV-like viruses in thalassemia patients and healthy individuals. J Clin Microbiol 43:3747–3754
Hu ZJ, Lang ZW, Zhou YS, Yan HP, Huang DZ, Chen WR, Luo ZX (2002) Clinicopathological study on TTV infection in hepatitis of unknown etiology. World J Gastroenterol 8:288–293
Huang LY, Oystein Jonassen T, Hungnes O, Grinde B (2001) High prevalence of TT virus-related DNA (90 %) and diverse viral genotypes in Norwegian blood donors. J Med Virol 64:381–386
Huang YH, Wu JC, Chiang TY, Chan YJ, Huo TI, Huang YS, Hwang SJ, Chang FY, Lee SD (2000) Detection and viral nucleotide sequence analysis of transfusion-transmitted virus infection in acute fulminant and non-fulminant hepatitis. J Viral Hepat 7:56–63
Hussain T, Manzoor S, Waheed Y, Tariq H, Hanif K (2012) Phylogenetic analysis of torque teno virus genome from Pakistani isolate and incidence of co-infection among HBV/HCV infected patients. Virol J 9:320
Ikeda H, Takasu M, Inoue K, Okamoto H, Miyakawa Y, Mayumi M (1999) Infection with an unenveloped DNA virus (TTV) in patients with acute or chronic liver disease of unknown etiology and in those positive for hepatitis C virus RNA. J Hepatol 30:205–212
Inami T, Konomi N, Arakawa Y, Abe K (2000) High prevalence of TT virus DNA in human saliva and semen. J Clin Microbiol 38:2407–2408
International Committee on Taxonomy of Viruses., King AMQ (2012) Virus taxonomy : classification and nomenclature of viruses : ninth report of the International Committee on Taxonomy of Viruses. Academic Press, London
Irshad M, Mandal K, Singh S, Agarwal SK (2010) Torque teno virus infection in hemodialysis patients in North India. Int Urol Nephrol 42:1077–1083
Irving WL, Ball JK, Berridge S, Curran R, Grabowska AM, Jameson CL, Neal KR, Ryder SD, Thomson BJ (1999) TT virus infection in patients with hepatitis C: frequency, persistence, and sequence heterogeneity. J Infect Dis 180:27–34
Ishikawa T, Hamano Y, Okamoto H (1999) Frequent detection of TT virus in throat swabs of pediatric patients. Infection 27:298
Iso K, Suzuki Y, Takayama M (2001) Mother-to-infant transmission of TT virus in Japan. Int J Gynaecol Obstet 75:11–19
Itoh M, Shimomura H, Fujioka S, Miyake M, Tsuji H, Ikeda F, Tsuji T (2001) High prevalence of TT virus in human bile juice samples: importance of secretion through bile into feces. Dig Dis Sci 46:457–462
Itoh N, Matsumura N, Ogi A, Nishide T, Imai Y, Kanai H, Ohno S (2000) High prevalence of herpes simplex virus type 2 in acute retinal necrosis syndrome associated with herpes simplex virus in Japan. Am J Ophthalmol 129:404–405
Jelcic I, Hotz-Wagenblatt A, Hunziker A, Zur Hausen H, de Villiers EM (2004) Isolation of multiple TT virus genotypes from spleen biopsy tissue from a Hodgkin’s disease patient: genome reorganization and diversity in the hypervariable region. J Virol 78:7498–7507
Jiang XJ, Luo KX, He HT (2000) Intrahepatic transfusion-transmitted virus detected by in situ hybridization in patients with liver diseases. J Viral Hepat 7:292–296
Jones MS, Kapoor A, Lukashov VV, Simmonds P, Hecht F, Delwart E (2005) New DNA viruses identified in patients with acute viral infection syndrome. J Virol 79:8230–8236
Kadayifci A, Guney C, Uygun A, Kubar A, Bagci S, Dagalp K (2001) Similar frequency of TT virus infection in patients with liver enzyme elevations and healthy subjects. Int J Clin Pract 55:434–436
Kakkola L, Bonden H, Hedman L, Kivi N, Moisala S, Julin J, Yla-Liedenpohja J, Miettinen S, Kantola K, Hedman K, Soderlund-Venermo M (2008) Expression of all six human Torque teno virus (TTV) proteins in bacteria and in insect cells, and analysis of their IgG responses. Virology 382:182–189
Kanda T, Yokosuka O (1999) Clinical feature of TTV-related hepatitis. Nihon Rinsho 57:1330–1334
Kato H, Mizokami M, Orito E, Ohno T, Hayashi K, Nakano T, Kato T, Tanaka Y, Sugauchi F, Mukaide M, Ueda R (2000) Lack of association between TTV viral load and aminotransferase levels in patients with hepatitis C or non-B-C. Scand J Infect Dis 32:259–262
Kazi A, Miyata H, Kurokawa K, Khan MA, Kamahora T, Katamine S, Hino S (2000) High frequency of postnatal transmission of TT virus in infancy. Arch Virol 145:535–540
Khudyakov YE, Cong ME, Nichols B, Reed D, Dou XG, Viazov SO, Chang J, Fried MW, Williams I, Bower W, Lambert S, Purdy M, Roggendorf M, Fields HA (2000) Sequence heterogeneity of TT virus and closely related viruses. J Virol 74:2990–3000
Kikuchi K, Miyakawa H, Abe K, Kako M, Katayama K, Fukushi S, Mishiro S (2000) Indirect evidence of TTV replication in bone marrow cells, but not in hepatocytes, of a subacute hepatitis/aplastic anemia patient. J Med Virol 61:165–170
Kincaid RP, Burke JM, Cox JC, de Villiers EM, Sullivan CS (2013) A human torque teno virus encodes a microRNA that inhibits interferon signaling. PLoS Pathog 9:e1003818
Leary TP, Erker JC, Chalmers ML, Desai SM, Mushahwar IK (1999) Improved detection systems for TT virus reveal high prevalence in humans, non-human primates and farm animals. J Gen Virol 80(Pt 8):2115–2120
Leppik L, Gunst K, Lehtinen M, Dillner J, Streker K, de Villiers EM (2007) In vivo and in vitro intragenomic rearrangement of TT viruses. J Virol 81:9346–9356
Lin HH, Kao JH, Lee PI, Chen DS (2002) Early acquisition of TT virus in infants: possible minor role of maternal transmission. J Med Virol 66:285–290
Lopez-Alcorocho JM, Mariscal LF, de Lucas S, Rodriguez-Inigo E, Casqueiro M, Castillo I, Bartolome J, Herrero M, Manzano ML, Pardo M, Carreno V (2000) Presence of TTV DNA in serum, liver and peripheral blood mononuclear cells from patients with chronic hepatitis. J Viral Hepat 7:440–447
Luo K, Zhang L (2001) Enteric transmission of transfusion-transmitted virus. Chin Med J (Engl) 114:1201–1204
Luo K, He H, Liu Z, Liu D, Xiao H, Jiang X, Liang W, Zhang L (2002) Novel variants related to TT virus distributed widely in China. J Med Virol 67:118–126
Maggi F, Fornai C, Morrica A, Casula F, Vatteroni ML, Marchi S, Ciccorossi P, Riente L, Pistello M, Bendinelli M (1999) High prevalence of TT virus viremia in Italian patients, regardless of age, clinical diagnosis, and previous interferon treatment. J Infect Dis 180:838–842
Maggi F, Fornai C, Vatteroni ML, Siciliano G, Menichetti F, Tascini C, Specter S, Pistello M, Bendinelli M (2001) Low prevalence of TT virus in the cerebrospinal fluid of viremic patients with central nervous system disorders. J Med Virol 65:418–422
Maggi F, Fornai C, Zaccaro L, Morrica A, Vatteroni ML, Isola P, Marchi S, Ricchiuti A, Pistello M, Bendinelli M (2001) TT virus (TTV) loads associated with different peripheral blood cell types and evidence for TTV replication in activated mononuclear cells. J Med Virol 64:190–194
Maggi F, Pistello M, Vatteroni M, Presciuttini S, Marchi S, Isola P, Fornai C, Fagnani S, Andreoli E, Antonelli G, Bendinelli M (2001) Dynamics of persistent TT virus infection, as determined in patients treated with alpha interferon for concomitant hepatitis C virus infection. J Virol 75:11999–12004
Maggi F, Fornai C, Tempestini E, Andreoli E, Lanini L, Vatteroni ML, Pistello M, Marchi S, Antonelli G, Bendinelli M (2003) Relationships between TT virus infection and hepatitis C virus response to interferon therapy in doubly infected patients. J Biol Regul Homeost Agents 17:176–182
Maggi F, Marchi S, Fornai C, Tempestini E, Andreoli E, Lanini L, Vatteroni ML, Bellini M, De Bortoli N, Costa F, Pistello M, Specter S, Bendinelli M (2003) Relationship of TT virus and Helicobacter pylori infections in gastric tissues of patients with gastritis. J Med Virol 71:160–165
Maggi F, Pifferi M, Fornai C, Andreoli E, Tempestini E, Vatteroni M, Presciuttini S, Marchi S, Pietrobelli A, Boner A, Pistello M, Bendinelli M (2003) TT virus in the nasal secretions of children with acute respiratory diseases: relations to viremia and disease severity. J Virol 77:2418–2425
Maggi F, Andreoli E, Lanini L, Fornai C, Vatteroni M, Pistello M, Presciuttini S, Bendinelli M (2005) Relationships between total plasma load of torquetenovirus (TTV) and TTV genogroups carried. J Clin Microbiol 43:4807–4810
Maggi F, Tempestini E, Lanini L, Andreoli E, Fornai C, Giannecchini S, Vatteroni M, Pistello M, Marchi S, Ciccorossi P, Specter S, Bendinelli M (2005) Blood levels of TT virus following immune stimulation with influenza or hepatitis B vaccine. J Med Virol 75:358–365
Maggi F, Andreoli E, Lanini L, Meschi S, Rocchi J, Fornai C, Vatteroni ML, Pistello M, Bendinelli M (2006) Rapid increase in total torquetenovirus (TTV) plasma viremia load reveals an apparently transient superinfection by a TTV of a novel group 2 genotype. J Clin Microbiol 44:2571–2574
Maggi F, Andreoli E, Riente L, Meschi S, Rocchi J, Delle Sedie A, Vatteroni ML, Ceccherini-Nelli L, Specter S, Bendinelli M (2007) Torquetenovirus in patients with arthritis. Rheumatology (Oxford) 46:885–886
Manni F, Rotola A, Caselli E, Bertorelle G, Di Luca D (2002) Detecting recombination in TT virus: a phylogenetic approach. J Mol Evol 55:563–572
Mariscal LF, Lopez-Alcorocho JM, Rodriguez-Inigo E, Ortiz-Movilla N, de Lucas S, Bartolome J, Carreno V (2002) TT virus replicates in stimulated but not in nonstimulated peripheral blood mononuclear cells. Virology 301:121–129
Martinez NM, Garcia F, Alvarez M, Bernal MC, Piedrola G, Hernandez J, Maroto MC (2000) TT virus DNA in serum, peripheral blood mononuclear cells and semen of patients infected by HIV. AIDS 14:1464–1466
Massau A, Martins C, Nachtigal GC, Araujo AB, Rossetti ML, Niel C, da Silva CM (2012) The high prevalence of Torque teno virus DNA in blood donors and haemodialysis patients in southern Brazil. Mem Inst Oswaldo Cruz 107:684–686
Matsubara H, Michitaka K, Horiike N, Yano M, Akbar SM, Torisu M, Onji M (2000) Existence of TT virus DNA in extracellular body fluids from normal healthy Japanese subjects. Intervirology 43:16–19
Matsubara H, Michitaka K, Horiike N, Kihana T, Yano M, Mori T, Onji M (2001) Existence of TT virus DNA and TTV-like mini virus DNA in infant cord blood: mother-to-neonatal transmission. Hepatol Res 21:280–287
Mi Z, Yuan X, Pei G, Wang W, An X, Zhang Z, Huang Y, Peng F, Li S, Bai C, Tong Y (2014) High-throughput sequencing exclusively identified a novel Torque teno virus genotype in serum of a patient with fatal fever. Virol Sin 29:112–118
Miyamoto M, Takahashi H, Sakata I, Adachi Y (2000) Hepatitis-associated aplastic anemia and transfusion-transmitted virus infection. Intern Med 39:1068–1070
Miyata H, Tsunoda H, Kazi A, Yamada A, Khan MA, Murakami J, Kamahora T, Shiraki K, Hino S (1999) Identification of a novel GC-rich 113-nucleotide region to complete the circular, single-stranded DNA genome of TT virus, the first human circovirus. J Virol 73:3582–3586
Moen EM, Sleboda J, Grinde B (2002) Real-time PCR methods for independent quantitation of TTV and TLMV. J Virol Methods 104:59–67
Moen EM, Sleboda J, Grinde B (2002) Serum concentrations of TT virus and TT virus-like mini virus in patients developing AIDS. AIDS 16:1679–1682
Moriyama M, Matsumura H, Shimizu T, Shioda A, Kaneko M, Miyazawa K, Miyata H, Tanaka N, Uchida T, Arakawa Y (2001) Histopathologic impact of TT virus infection on the liver of type C chronic hepatitis and liver cirrhosis in Japan. J Med Virol 64:74–81
Morrica A, Maggi F, Vatteroni ML, Fornai C, Pistello M, Ciccorossi P, Grassi E, Gennazzani A, Bendinelli M (2000) TT virus: evidence for transplacental transmission. J Infect Dis 181:803–804
Mushahwar IK, Erker JC, Muerhoff AS, Leary TP, Simons JN, Birkenmeyer LG, Chalmers ML, Pilot-Matias TJ, Dexai SM (1999) Molecular and biophysical characterization of TT virus: evidence for a new virus family infecting humans. Proc Natl Acad Sci USA 96:3177–3182
Naoumov NV, Petrova EP, Thomas MG, Williams R (1998) Presence of a newly described human DNA virus (TTV) in patients with liver disease. Lancet 352:195–197
Niel C, Diniz-Mendes L, Devalle S (2005) Rolling-circle amplification of Torque teno virus (TTV) complete genomes from human and swine sera and identification of a novel swine TTV genogroup. J Gen Virol 86:1343–1347
Ninomiya M, Nishizawa T, Takahashi M, Lorenzo FR, Shimosegawa T, Okamoto H (2007) Identification and genomic characterization of a novel human torque teno virus of 3.2 kb. J Gen Virol 88:1939–1944
Ninomiya M, Takahashi M, Shimosegawa T, Okamoto H (2007) Analysis of the entire genomes of fifteen torque teno midi virus variants classifiable into a third group of genus Anellovirus. Arch Virol 152:1961–1975
Ninomiya M, Takahashi M, Nishizawa T, Shimosegawa T, Okamoto H (2008) Development of PCR assays with nested primers specific for differential detection of three human anelloviruses and early acquisition of dual or triple infection during infancy. J Clin Microbiol 46:507–514
Nishiguchi S, Enomoto M, Shiomi S, Tanaka M, Fukuda K, Tamori A, Tanaka T, Takeda T, Seki S, Yano Y, Otani S, Kuroki T (2000) TT virus infection in patients with chronic liver disease of unknown etiology. J Med Virol 62:392–398
Nishiyama S, Dutia BM, Stewart JP, Meredith AL, Shaw DJ, Simmonds P, Sharp CP (2014) Identification of novel anelloviruses with broad diversity in UK rodents. J Gen Virol 95:1544–1553
Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M (1997) A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun 241:92–97
Nishizawa T, Okamoto H, Tsuda F, Aikawa T, Sugai Y, Konishi K, Akahane Y, Ukita M, Tanaka T, Miyakawa Y, Mayumi M (1999) Quasispecies of TT virus (TTV) with sequence divergence in hypervariable regions of the capsid protein in chronic TTV infection. J Virol 73:9604–9608
Nuanualsuwan S, Cliver DO (2002) Pretreatment to avoid positive RT-PCR results with inactivated viruses. J Virol Methods 104:217–225
Ohto H, Ujiie N, Takeuchi C, Sato A, Hayashi A, Ishiko H, Nishizawa T, Okamoto H (2002) TT virus infection during childhood. Transfusion 42:892–898
Okamoto H, Akahane Y, Ukita M, Fukuda M, Tsuda F, Miyakawa Y, Mayumi M (1998) Fecal excretion of a nonenveloped DNA virus (TTV) associated with posttransfusion non-A-G hepatitis. J Med Virol 56:128–132
Okamoto H, Nishizawa T, Kato N, Ukita M, Ikeda H, Iizuka H, Miyakawa Y, Mayumi M (1998) Molecular cloning and characterization of a novel DNA virus (TTV) associated with posttransfusion hepatitis of unknown etiology. Hepatol Res 10:1–16
Okamoto H, Kato N, Iizuka H, Tsuda F, Miyakawa Y, Mayumi M (1999) Distinct genotypes of a nonenveloped DNA virus associated with posttransfusion non-A to G hepatitis (TT virus) in plasma and peripheral blood mononuclear cells. J Med Virol 57:252–258
Okamoto H, Nishizawa T, Tawara A, Takahashi M, Kishimoto J, Sai T, Sugai Y (2000) TT virus mRNAs detected in the bone marrow cells from an infected individual. Biochem Biophys Res Commun 279:700–707
Okamoto H, Ukita M, Nishizawa T, Kishimoto J, Hoshi Y, Mizuo H, Tanaka T, Miyakawa Y, Mayumi M (2000) Circular double-stranded forms of TT virus DNA in the liver. J Virol 74:5161–5167
Okamoto H, Nishizawa T, Takahashi M, Asabe S, Tsuda F, Yoshikawa A (2001) Heterogeneous distribution of TT virus of distinct genotypes in multiple tissues from infected humans. Virology 288:358–368
Okamoto H, Nishizawa T, Takahashi M, Tawara A, Peng Y, Kishimoto J, Wang Y (2001) Genomic and evolutionary characterization of TT virus (TTV) in tupaias and comparison with species-specific TTVs in humans and non-human primates. J Gen Virol 82:2041–2050
Okamura A, Yoshioka M, Kubota M, Kikuta H, Ishiko H, Kobayashi K (1999) Detection of a novel DNA virus (TTV) sequence in peripheral blood mononuclear cells. J Med Virol 58:174–177
Okamura A, Yoshioka M, Kikuta H, Kubota M, Ma X, Hayashi A, Ishiko H, Kobayashi K (2000) Detection of TT virus sequences in children with liver disease of unknown etiology. J Med Virol 62:104–108
Okonechnikov K, Golosova O, Fursov M (2012) Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics 28:1166–1167
Osiowy C, Sauder C (2000) Detection of TT virus in human hair and skin. Hepatol Res 16:155–162
Parras-Molto M, Suarez-Rodriguez P, Eguia A, Aguirre-Urizar JM, Lopez-Bueno A (2014) Genome sequence of two novel species of torque teno minivirus from the human oral cavity. Genome Announc 2
Peng YH, Nishizawa T, Takahashi M, Ishikawa T, Yoshikawa A, Okamoto H (2002) Analysis of the entire genomes of thirteen TT virus variants classifiable into the fourth and fifth genetic groups, isolated from viremic infants. Arch Virol 147:21–41
Pifferi M, Maggi F, Andreoli E, Lanini L, Marco ED, Fornai C, Vatteroni ML, Pistello M, Ragazzo V, Macchia P, Boner A, Bendinelli M (2005) Associations between nasal torquetenovirus load and spirometric indices in children with asthma. J Infect Dis 192:1141–1148
Pifferi M, Maggi F, Caramella D, De Marco E, Andreoli E, Meschi S, Macchia P, Bendinelli M, Boner AL (2006) High torquetenovirus loads are correlated with bronchiectasis and peripheral airflow limitation in children. Pediatr Infect Dis J 25:804–808
Pinho-Nascimento CA, Leite JP, Niel C, Diniz-Mendes L (2011) Torque teno virus in fecal samples of patients with gastroenteritis: prevalence, genogroups distribution, and viral load. J Med Virol 83:1107–1111
Pisani G, Cristiano K, Wirz M, Bisso G, Beneduce F, Morace G, Rapicetta M, Gentili G (1999) Prevalence of TT virus in plasma pools and blood products. Br J Haematol 106:431–435
Pollicino T, Raffa G, Squadrito G, Costantino L, Cacciola I, Brancatelli S, Alafaci C, Florio MG, Raimondo G (2003) TT virus has a ubiquitous diffusion in human body tissues: analyses of paired serum and tissue samples. J Viral Hepat 10:95–102
Poovorawan Y, Theamboonlers A, Vimolket T, Jantaradsamee P, Kaew-in N, Hirsch P (1999) Detection of TTV in peripheral blood mononuclear cells of intravenous drug users. Tohoku J Exp Med 188:47–54
Poovorawan Y, Tangkijvanich P, Theamboonlers A, Hirsch P (2001) Transfusion transmissible virus TTV and its putative role in the etiology of liver disease. Hepatogastroenterology 48:256–260
Prescott LE, MacDonald DM, Davidson F, Mokili J, Pritchard DI, Arnot DE, Riley EM, Greenwood BM, Hamid S, Saeed AA, McClure MO, Smith DB, Simmonds P (1999) Sequence diversity of TT virus in geographically dispersed human populations. J Gen Virol 80(Pt 7):1751–1758
Rivanera D, Lozzi MA, Idili C, Lilli D (2009) Prevalence of TT virus infection in Italian dialysis patients. Pathol Biol (Paris) 57:97–100
Rocchi J, Ricci V, Albani M, Lanini L, Andreoli E, Macera L, Pistello M, Ceccherini-Nelli L, Bendinelli M, Maggi F (2009) Torquetenovirus DNA drives proinflammatory cytokines production and secretion by immune cells via toll-like receptor 9. Virology 394:235–242
Rodriguez-Inigo E, Casqueiro M, Bartolome J, Ortiz-Movilla N, Lopez-Alcorocho JM, Herrero M, Manzarbeitia F, Oliva H, Carreno V (2000) Detection of TT virus DNA in liver biopsies by in situ hybridization. Am J Pathol 156:1227–1234
Roperto S, Paciello O, Paolini F, Pagnini U, Palma E, Di Palo R, Russo V, Roperto F, Venuti A (2009) Short communication: detection of human Torque teno virus in the milk of water buffaloes (Bubalus bubalis). J Dairy Sci 92:5928–5932
Ross RS, Viazov S, Runde V, Schaefer UW, Roggendorf M (1999) Detection of TT virus DNA in specimens other than blood. J Clin Virol 13:181–184
Saback FL, Gomes SA, de Paula VS, da Silva RR, Lewis-Ximenez LL, Niel C (1999) Age-specific prevalence and transmission of TT virus. J Med Virol 59:318–322
Safadi R, Or R, Ilan Y, Naparstek E, Nagler A, Klein A, Ketzinel-Gilaad M, Ergunay K, Danon D, Shouval D, Galun E (2001) Lack of known hepatitis virus in hepatitis-associated aplastic anemia and outcome after bone marrow transplantation. Bone Marrow Transplant 27:183–190
Sampietro M, Tavazzi D, Martinez di Montemuros F, Cerino M, Zatelli S, Lunghi G, Orlandi A, Fargion S, Fiorelli G, Cappellini MD (2001) TT virus infection in adult beta-thalassemia major patients. Haematologica 86:39–43
Schroter M, Polywka S, Zollner B, Schafer P, Laufs R, Feucht HH (2000) Detection of TT virus DNA and GB virus type C/Hepatitis G virus RNA in serum and breast milk: determination of mother-to-child transmission. J Clin Microbiol 38:745–747
Schroter M, Laufs R, Zollner B, Knodler B, Schafer P, Feucht HH (2003) A novel DNA virus (SEN) among patients on maintenance hemodialysis: prevalence and clinical importance. J Clin Virol 27:69–73
Shackelton LA, Parrish CR, Holmes EC (2006) Evolutionary basis of codon usage and nucleotide composition bias in vertebrate DNA viruses. J Mol Evol 62:551–563
Shibayama T, Masuda G, Ajisawa A, Takahashi M, Nishizawa T, Tsuda F, Okamoto H (2001) Inverse relationship between the titre of TT virus DNA and the CD4 cell count in patients infected with HIV. AIDS 15:563–570
Sospedra M, Zhao Y, zur Hausen H, Muraro PA, Hamashin C, de Villiers EM, Pinilla C, Martin R (2005) Recognition of conserved amino acid motifs of common viruses and its role in autoimmunity. PLoS Pathog 1:e41
Spandole S, Tudor A, Berca LM, Adascalului M, Niculae OM, Cimponeriu D, Mihaescu G (2013) Torque teno viruses DNA found in meat products4. Curr Opin Biotechnol 24, Suppl 1:S94–S95
Sugiyama K, Goto K, Ando T, Mizutani F, Terabe K, Kawabe Y, Wada Y (1999) Route of TT virus infection in children. J Med Virol 59:204–207
Szladek G, Juhasz A, Kardos G, Szoke K, Major T, Sziklai I, Tar I, Marton I, Konya J, Gergely L, Szarka K (2005) High co-prevalence of genogroup 1 TT virus and human papillomavirus is associated with poor clinical outcome of laryngeal carcinoma. J Clin Pathol 58:402–405
Takahashi K, Hijikata M, Samokhvalov EI, Mishiro S (2000) Full or near full length nucleotide sequences of TT virus variants (Types SANBAN and YONBAN) and the TT virus-like mini virus. Intervirology 43:119–123
Takahashi K, Iwasa Y, Hijikata M, Mishiro S (2000) Identification of a new human DNA virus (TTV-like mini virus, TLMV) intermediately related to TT virus and chicken anemia virus. Arch Virol 145:979–993
Takahashi M, Asabe S, Gotanda Y, Kishimoto J, Tsuda F, Okamoto H (2002) TT virus is distributed in various leukocyte subpopulations at distinct levels, with the highest viral load in granulocytes. Biochem Biophys Res Commun 290:242–248
Takayama S, Miura T, Matsuo S, Taki M, Sugii S (1999) Prevalence and persistence of a novel DNA TT virus (TTV) infection in Japanese haemophiliacs. Br J Haematol 104:626–629
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729
Tanaka H, Okamoto H, Luengrojanakul P, Chainuvati T, Tsuda F, Tanaka T, Miyakawa Y, Mayumi M (1998) Infection with an unenveloped DNA virus (TTV) associated with posttransfusion non-A to G hepatitis in hepatitis patients and healthy blood donors in Thailand. J Med Virol 56:234–238
Tanaka M, Nishiguchi S, Tanaka T, Enomoto M, Takeda T, Shiomi S, Kuroki T, Otani S (1999) Prevalence of TT virus in patients with fulminant hepatic failure in Japan. J Gastroenterol 34:589–593
Tanaka Y, Hayashi J, Ariyama I, Furusyo N, Etoh Y, Kashiwagi S (2000) Seroepidemiology of TT virus infection and relationship between genotype and liver damage. Dig Dis Sci 45:2214–2220
Tanaka Y, Primi D, Wang RY, Umemura T, Yeo AE, Mizokami M, Alter HJ, Shih JW (2001) Genomic and molecular evolutionary analysis of a newly identified infectious agent (SEN virus) and its relationship to the TT virus family. J Infect Dis 183:359–367
Tokita H, Murai S, Kamitsukasa H, Yagura M, Harada H, Takahashi M, Okamoto H (2002) High TT virus load as an independent factor associated with the occurrence of hepatocellular carcinoma among patients with hepatitis C virus-related chronic liver disease. J Med Virol 67:501–509
Touinssi M, Gallian P, Biagini P, Attoui H, Vialettes B, Berland Y, Tamalet C, Dhiver C, Ravaux I, De Micco P, De Lamballerie X (2001) TT virus infection: prevalence of elevated viraemia and arguments for the immune control of viral load. J Clin Virol 21:135–141
Tuveri R, Jaffredo F, Lunel F, Nalpa B, Pol S, Feray C, Marcellin P, Thibault V, Delagneau JF, Opolon P, Scarpa B, Brechot C, Thiers V (2000) Impact of TT virus infection in acute and chronic, viral- and non viral-related liver diseases. J Hepatol 33:121–127
Tyagi AK, Pradier A, Baumer O, Uppugunduri CR, Huezo-Diaz P, Posfay-Barbe KM, Roosnek E, Ansari M (2013) Validation of SYBR Green based quantification assay for the detection of human Torque Teno virus titers from plasma. Virol J 10:191
Ukita M, Okamoto H, Kato N, Miyakawa Y, Mayumi M (1999) Excretion into bile of a novel unenveloped DNA virus (TT virus) associated with acute and chronic non-A-G hepatitis. J Infect Dis 179:1245–1248
Umemura T, Alter HJ, Tanaka E, Yeo AE, Shih JW, Orii K, Matsumoto A, Yoshizawa K, Kiyosawa K (2001) Association between SEN virus infection and hepatitis C in Japan. J Infect Dis 184:1246–1251
Umemura T, Tanaka E, Ostapowicz G, Brown KE, Heringlake S, Tassopoulos NC, Wang RY, Yeo AE, Shih JW, Orii K, Young NS, Hatzakis A, Manns MP, Lee WM, Kiyosawa K, Alter HJ (2003) Investigation of SEN virus infection in patients with cryptogenic acute liver failure, hepatitis-associated aplastic anemia, or acute and chronic non-A-E hepatitis. J Infect Dis 188:1545–1552
Vaidya SR, Chitambar SD, Arankalle VA (2002) Polymerase chain reaction-based prevalence of hepatitis A, hepatitis E and TT viruses in sewage from an endemic area. J Hepatol 37:131–136
Vasconcelos HC, Gomes SA, Cataldo M, Niel C (2003) Prevalence and genetic diversity of TT virus genotype 21 (YONBAN virus) in Brazil. Arch Virol 148:517–529
Vasilyev EV, Trofimov DY, Tonevitsky AG, Ilinsky VV, Korostin DO, Rebrikov DV (2009) Torque Teno Virus (TTV) distribution in healthy Russian population. Virol J 6:134
Verani M, Casini B, Battistini R, Pizzi F, Rovini E, Carducci A (2006) One-year monthly monitoring of Torque teno virus (TTV) in river water in Italy. Water Sci Technol 54:191–195
Verani M, Bigazzi R, Carducci A (2014) Viral contamination of aerosol and surfaces through toilet use in health care and other settings. Am J Infect Control 42:758–762
Worobey M (2000) Extensive homologous recombination among widely divergent TT viruses. J Virol 74:7666–7670
Yokoyama H, Yasuda J, Okamoto H, Iwakura Y (2002) Pathological changes of renal epithelial cells in mice transgenic for the TT virus ORF1 gene. J Gen Virol 83:141–150
Yu Q, Shiramizu B, Nerurkar VR, Hu N, Shikuma CM, Melish ME, Cascio K, Imrie A, Lu Y, Yanagihara R (2002) TT virus: preferential distribution in CD19(+) peripheral blood mononuclear cells and lack of viral integration. J Med Virol 66:276–284
Zhang H, Gao S, Lercher MJ, Hu S, Chen WH (2012) EvolView, an online tool for visualizing, annotating and managing phylogenetic trees. Nucleic Acids Res 40:W569–W572
Zheng MY, Lin Y, Li DJ, Ruan HB, Chen Y, Wu TT (2010) TTV and HPV co-infection in cervical smears of patients with cervical lesions in littoral of Zhejiang province. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 24:110–112
Zhong S, Yeo W, Lin CK, Lin XR, Tang MW, Johnson PJ (2001) Quantitative and genotypic analysis of TT virus infection in Chinese blood donors. Transfusion 41:1001–1007
Zhong S, Yeo W, Tang MW, Lin XR, Mo F, Ho WM, Hui P, Johnson PJ (2001) Gross elevation of TT virus genome load in the peripheral blood mononuclear cells of cancer patients. Ann N Y Acad Sci 945:84–92
Zhong S, Yeo W, Tang M, Liu C, Lin XR, Ho WM, Hui P, Johnson PJ (2002) Frequent detection of the replicative form of TT virus DNA in peripheral blood mononuclear cells and bone marrow cells in cancer patients. J Med Virol 66:428–434
zur Hausen H, de Villiers EM (2005) Virus target cell conditioning model to explain some epidemiologic characteristics of childhood leukemias and lymphomas. Int J Cancer 115:1–5
Acknowledgments
This paper was written in the framework of project PN 12 48, funded by the Romanian Ministry of Education and granted to the National Institute of Research and Development for Food Bioresources.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Spandole, S., Cimponeriu, D., Berca, L.M. et al. Human anelloviruses: an update of molecular, epidemiological and clinical aspects. Arch Virol 160, 893–908 (2015). https://doi.org/10.1007/s00705-015-2363-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-015-2363-9