Abstract
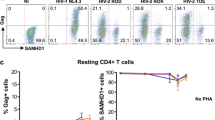
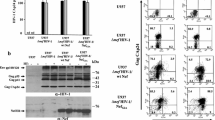
A central feature of HIV-1 infection is the inability of entering virus to integrate into chromosomes of resting T lymphocytes unless they are mitogenically activated. In contrast, SIVpbj1.9 replicates in initially resting T lymphocytes by activating infected cells. Previous reports have shown that a difference in Nef-mediated T cell activation between HIV-1 and SIVpbj1.9 plays a critical role in the differing abilities of these viruses to replicate in resting lymphocytes. However, the molecular details of these differences are still unclear. Here, we show that infection with a chimeric virus, HSIVnef, which harbors the 5′ 308 nucleotides of SIVpbj1.9 nef in place of the 5′ 221 nucleotides of HIV-1 nef in the HIV-1 proviral backbone, resulted in integration of the provirus into host chromosomes without mitogenic activation and thereby replication in resting human PBMCs (hPBMCs). These results indicate that Nef is an essential viral determinant for the integration of provirus into host chromosomes in resting T cells. Using the yeast two-hybrid system, we identified integrase interactor-1 (INI1/SMARCB1) as a cellular factor that is involved in the integration process via interaction with Nef. Although INI1 interacted with both SIVpbj1.9 and HIV-1 Nefs, SIVpbj1.9 Nef, but not HIV-1 Nef, enhanced proviral integration into host DNA. Furthermore, mutational analysis revealed that the basic-amino-acid-rich amino-terminal domain in SIVpbj1.9 Nef is crucial for interaction with INI1 and virus replication in resting hPBMCs. Taken together, these data indicate that Nef is a critical viral protein for incorporating nascent proviral DNA into host chromosomes in resting PBMCs and that this occurs through interaction with INI1. This elucidates the basis for replication of the integrated provirus when the host cell is in a resting state.






Similar content being viewed by others
References
Pierson TC, Zhou Y, Kieffer TL, Ruff CT, Buck C et al (2002) Molecular characterization of preintegration latency in human immunodeficiency virus type 1 infection. J Virol 76:8518–8531
Strebel K, Luban J, Jeang KT (2009) Human cellular restriction factors that target HIV-1 replication. BMC Med 7:48
Gao WY, Cara A, Gallo RC, Lori F (1993) Low levels of deoxynucleotides in peripheral blood lymphocytes: a strategy to inhibit human immunodeficiency virus type 1 replication. Proc Natl Acad Sci USA 90:8925–8928
Malim MH (2009) APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos Trans R Soc Lond B Biol Sci 364:675–687
Goila-Gaur R, Khan MA, Miyagi E, Strebel K (2009) Differential sensitivity of “old” versus “new” APOBEC3G to human immunodeficiency virus type 1 vif. J Virol 83:1156–1160
Chiu YL, Soros VB, Kreisberg JF, Stopak K, Yonemoto W et al (2005) Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature 435:108–114
Goila-Gaur R, Strebel K (2008) HIV-1 Vif, APOBEC, and intrinsic immunity. Retrovirology 5:51
Bukrinsky MI, Stanwick TL, Dempsey MP, Stevenson M (1991) Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science 254:423–427
Bukrinsky MI, Sharova N, Dempsey MP, Stanwick TL, Bukrinskaya AG et al (1992) Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci USA 89:6580–6584
Spina CA, Guatelli JC, Richman DD (1995) Establishment of a stable, inducible form of human immunodeficiency virus type 1 DNA in quiescent CD4 lymphocytes in vitro. J Virol 69:2977–2988
Stevenson M, Stanwick TL, Dempsey MP, Lamonica CA (1990) HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J 9:1551–1560
Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A et al (1990) HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61:213–222
Manganaro L, Lusic M, Gutierrez MI, Cereseto A, Del Sal G et al (2010) Concerted action of cellular JNK and Pin1 restricts HIV-1 genome integration to activated CD4+ T lymphocytes. Nat Med 16:329–333
Dewhurst S, Embretson JE, Anderson DC, Mullins JI, Fultz PN (1990) Sequence analysis and acute pathogenicity of molecularly cloned SIVSMM-PBj14. Nature 345:636–640
Fultz PN, McClure HM, Anderson DC, Switzer WM (1989) Identification and biologic characterization of an acutely lethal variant of simian immunodeficiency virus from sooty mangabeys (SIV/SMM). AIDS Res Hum Retroviruses 5:397–409
Fultz PN (1991) Replication of an acutely lethal simian immunodeficiency virus activates and induces proliferation of lymphocytes. J Virol 65:4902–4909
Du Z, Lang SM, Sasseville VG, Lackner AA, Ilyinskii PO et al (1995) Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell 82:665–674
Stephens EB, Mukherjee S, Liu ZQ, Sheffer D, Lamb-Wharton R et al (1998) Simian-human immunodeficiency virus (SHIV) containing the nef/long terminal repeat region of the highly virulent SIVsmmPBj14 causes PBj-like activation of cultured resting peripheral blood mononuclear cells, but the chimera showed No increase in virulence. J Virol 72:5207–5214
Park IW, He JJ (2013) HIV-1 Nef-mediated T-cell activation and chemotaxis are decoupled using a HIV-1/SIVpbj1.9. Chimeric nef variant. Arch Virol 158:845–852
Schibeci SD, Clegg AO, Biti RA, Sagawa K, Stewart GJ et al (2000) HIV-Nef enhances interleukin-2 production and phosphatidylinositol 3-kinase activity in a human T cell line. AIDS 14:1701–1707
Schrager JA, Marsh JW (1999) HIV-1 Nef increases T cell activation in a stimulus-dependent manner. Proc Natl Acad Sci USA 96:8167–8172
Wang JF, Park IW, Groopman JE (2000) Stromal cell-derived factor-1alpha stimulates tyrosine phosphorylation of multiple focal adhesion proteins and induces migration of hematopoietic progenitor cells: roles of phosphoinositide-3 kinase and protein kinase C. Blood 95:2505–2513
Margol AS, Judkins AR (2014) Pathology and diagnosis of SMARCB1-deficient tumors. Cancer Genet pii S2210–7762(14):00155
Kalpana GV, Marmon S, Wang W, Crabtree GR, Goff SP (1994) Binding and stimulation of HIV-1 integrase by a human homolog of yeast transcription factor SNF5. Science 266:2002–2006
Ertel W, Kremer JP, Kenney J, Steckholzer U, Jarrar D et al (1995) Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood 85:1341–1347
Park IW, Myrick K, Sodroski J (1994) Effects of vif mutations on cell-free infectivity and replication of simian immunodeficiency virus. J Acquir Immune Defic Syndr 7:1228–1236
Wu P, Phillips MI, Bui J, Terwilliger EF (1998) Adeno-associated virus vector-mediated transgene integration into neurons and other nondividing cell targets. J Virol 72:5919–5926
Fagan R, Flint KJ, Jones N (1994) Phosphorylation of E2F-1 modulates its interaction with the retinoblastoma gene product and the adenoviral E4 19 kDa protein. Cell 78:799–811
Alkhatib G, Liao F, Berger EA, Farber JM, Peden KW (1997) A few SIV co-receptor, STRL33. Nature 388:238
Farzan M, Choe H, Martin K, Marcon L, Hofmann W et al (1997) Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med 186:405–411
Manninen A, Huotari P, Hiipakka M, Renkema GH, Saksela K (2001) Activation of NFAT- dependent gene expression by Nef: conservation among divergent Nef alleles, dependence on SH3 binding and membrane association, and cooperation with protein kinase C-theta. J Virol 75:3034–3037
Moarefi I, LaFevre-Bernt M, Sicheri F, Huse M, Lee CH, Kuriyan J, Miller WT (1997) Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature 385:650–653
Saksela K, Cheng G, Baltimore D (1995) Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J 14:484–491
Zhou Y, Zhang H, Siliciano JD, Siliciano RF (2005) Kinetics of human immunodeficiency virus type 1 decay following entry into resting CD4+ T cells. J Virol 79:2199–2210
Swiggard WJ, Baytop C, Yu JJ, Dai J, Li C et al (2005) Human immunodeficiency virus type 1 can establish latent infection in resting CD4+ T cells in the absence of activating stimuli. J Virol 79:14179–14188
Cameron PU, Saleh S, Sallmann G, Solomon A, Wightman F et al (2010) Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton. Proc Natl Acad Sci USA 107:16934–16939
Colin L, Van Lint C (2009) Molecular control of HIV-1 postintegration latency: implications for the development of new therapeutic strategies. Retrovirology 6:111
Pierson TC, Kieffer TL, Ruff CT, Buck C, Gange SJ et al (2002) Intrinsic stability of episomal circles formed during human immunodeficiency virus type 1 replication. J Virol 76:4138–4144
Simmons A, Aluvihare V, McMichael A (2001) Nef triggers a transcriptional program in T cells imitating single-signal T cell activation and inducing HIV virulence mediators. Immunity 14:763–777
Keppler OT, Tibroni N, Venzke S, Rauch S, Fackler OT (2006) Modulation of specific surface receptors and activation sensitization in primary resting CD4+ T lymphocytes by the Nef protein of HIV-1. J Leukoc Biol 79:616–627
Franchini G, Robert-Guroff M, Ghrayeb J, Chang NT, Wong-Staal F (1986) Cytoplasmic localization of the HTLV-III 3′ orf protein in cultured T cells. Virology 155:593–599
Yu G, Felsted RL (1992) Effect of myristoylation on p27 nef subcellular distribution and suppression of HIV-LTR transcription. Virology 187:46–55
Craig E, Zhang ZK, Davies KP, Kalpana GV (2002) A masked NES in INI1/hSNF5 mediates hCRM1-dependent nuclear export: implications for tumorigenesis. EMBO J 21:31–42
Turelli P, Doucas V, Craig E, Mangeat B, Klages N et al (2001) Cytoplasmic recruitment of INI1 and PML on incoming HIV preintegration complexes: interference with early steps of viral replication. Mol Cell 7:1245–1254
Aiken C, Trono D (1995) Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol 69:5048–5056
Chowers MY, Pandori MW, Spina CA, Richman DD, Guatelli JC (1995) The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology 212:451–457
Schwartz O, Marechal V, Danos O, Heard JM (1995) Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol 69:4053–4059
Qi M, Aiken C (2008) Nef enhances HIV-1 infectivity via association with the virus assembly complex. Virology 373:287–297
Ma W, Mishra S, Gajanayaka N, Angel JB, Kumar A (2009) HIV-1 Nef inhibits lipopolysaccharide-induced IL-12p40 expression by inhibiting JNK-activated NFkappaB in human monocytic cells. J Biol Chem 284:7578–7587
Mangino G, Percario ZA, Fiorucci G, Vaccari G, Manrique S et al (2007) In vitro treatment of human monocytes/macrophages with myristoylated recombinant Nef of human immunodeficiency virus type 1 leads to the activation of mitogen-activated protein kinases, IkappaB kinases, and interferon regulatory factor 3 and to the release of beta interferon. J Virol 81:2777–2791
Lee SB, Park J, Jung JU, Chung J (2005) Nef induces apoptosis by activating JNK signaling pathway and inhibits NF-kappaB-dependent immune responses in Drosophila. J Cell Sci 118:1851–1859
Varin A, Manna SK, Quivy V, Decrion AZ, Van Lint C et al (2003) Exogenous Nef protein activates NF-kappa B, AP-1, and c-Jun N-terminal kinase and stimulates HIV transcription in promonocytic cells. Role in AIDS pathogenesis. J Biol Chem 278:2219–2227
Schrager JA, Der Minassian V, Marsh JW (2002) HIV Nef increases T cell ERK MAP kinase activity. J Biol Chem 277:6137–6142
Acknowledgments
We thank Drs. Ganjam V. Kalpana (Albert Einstein College of Medicine), Alan Engelman (Dana Farber Cancer Institute), and John A. A. Ladias (Harvard Medical School) for experimental materials and advice. This work was supported by the David Geffen Research Fellowship for AIDS Research (D. P.) and NIH/NIDDK R01 DK099055 (I.-W. P).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Pyeon, D., Park, IW. Interaction between Nef and INI1/SMARCB1 augments replicability of HIV-1 in resting human peripheral blood mononuclear cells. Arch Virol 160, 727–737 (2015). https://doi.org/10.1007/s00705-014-2315-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-014-2315-9