Abstract
Bokeloh bat lyssavirus (BBLV) was found in Myotis nattereri for the first time in northeastern France in July 2012. The complete genome sequence of the virus from the infected Natterer’s bat was determined by whole-genome sequencing and compared to that of the first BBLV strain isolated in 2010 in Germany and with those of all currently identified lyssaviruses. The French isolate [KC169985] showed 98.7 % nucleotide sequence identity to the German BBLV strain [JF311903]. Several organs of the infected French bat were examined by classical rabies diagnostic methods: fluorescent antibody test, cell culture inoculation test and RT-qPCR. Antigen, infectious virus and high viral RNA levels were found in both the brain and salivary glands. Traces of genomic RNA were detected in the bladder, kidney and lung tissue. The results of an investigation of the distribution of lyssaviruses with the detection of infectious virus in the salivary glands suggest a possible mode of transmission of the virus.
Similar content being viewed by others
Introduction
Rabies in bats is documented worldwide, and the first case in Europe was reported in 1954 [18]. Although bats have been shown since the 1990s to be infected with viruses responsible for zoonotic viral diseases, such as Nipah virus and Hendra virus [12], bat rabies is the oldest known zoonotic disease and is currently acknowledged as the main bat-associated disease [26]. Rabies disease is an encephalitis caused by rabies viruses and is transmissible to all mammals. Due to the pathogenesis of rabies, and as no clinical sign or postmortem lesion can be considered pathognomonic in animals suspected of having rabies, identification of rabies virus infections has to rely on laboratory testing. Bat rabies is propagated by viruses in the family Rhabdoviridae, genus Lyssavirus. There are currently twelve recognised members of the genus Lyssavirus [8]: rabies virus (RABV), Lagos bat virus (LBV), Mokola virus (MOKV), Duvenhage virus (DUVV), European bat lyssavirus type 1 (EBLV-1) European bat lyssavirus type 2 (EBLV-2), Australian bat lyssavirus (ABLV), Irkut virus (IRKV), Aravan virus (ARAV), Khujand virus (KHUV), West Caucasian bat virus (WCBV) and Shimoni bat virus. MOKV is the only lyssavirus that was never isolated from bats. Two novel lyssaviruses have been identified recently in bats but have not yet been classified: Ikoma bat lyssavirus (IKOV), discovered in 2009 in an African civet [15], and Bokeloh bat lyssavirus (BBLV) identified in a Natterer’s bat (Myotis nattereri) in Germany in 2010 [5].
In Europe, bat rabies is caused by four types of lyssavirus: EBLV-1, EBLV-2, WCBV and the new member, BBLV [16]. From 1977 to 2011, a total of 961 cases of bat rabies were reported throughout Europe (http://www.who-rabies-bulletin.org). Of these, 95 % of cases were attributed to EBLV-1, 23 to EBLV-2, one to WCBV in Miniopterus schreibersii in southwestern Russia in 2002 [11], and one to BBLV in M. nattereri [5]. Although bat rabies has been principally reported in serotine bats (Eptesicus serotinus), with 99 % of cases associated with EBLV-1, other species—such as Daubenton’s bats (Myotis daubentonii, n=18) and pond bats (Myotis dasycneme, n=6)—have been shown to be infected with EBLV-2 [16]. To date, WCBV and BBLV have only been isolated once in Europe.
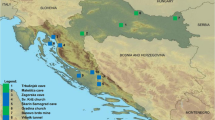
In France, bat rabies has been under surveillance since 1989, with the first case reported in a serotine bat (Eptesicus serotinus) infected with EBLV-1. In 2000, bat rabies surveillance was improved with the consolidation of the network and involvement of local veterinary services in addition to SFEPM, the French national bat conservation network [20]. Since its inception in 1989, the bat rabies surveillance network has reported 61 cases of EBLV-1 infection in 2,457 bats tested [21]. All 61 cases involved the same species (E. serotinus) in different areas of the country (Fig. 1). To complement passive surveillance, field studies carried out in France since 2004 involving 18 of the 45 bat species reported in Europe have detected rabies antibodies in six indigenous bat species, mainly E. serotinus and Myotis myotis, particularly in central and southwestern France [22].
Here, we report for the first time in France the isolation of BBLV in M. nattereri [23]. The presence of the virus in different organs collected from the infected bat was assessed using routine rabies diagnostic techniques (fluorescent antibody test [FAT] and rabies tissue culture infection test [RTCIT]) [17] and quantitative reverse transcription polymerase chain reaction (RT-qPCR) [7, 32].
Materials and methods
Case study
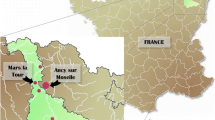
On June 4, 2012, an apparently healthy bat was captured during the night in a forest near Hémilly in northeastern France. The bat was equipped with a radio transmitter for a local bat inventory and then released. Bat specialists identified it as M. nattereri using morphological criteria [3]. The caught bat did not exhibit any signs of clinical rabies. The nulliparous adult was not aggressive and exhibited normal behaviour. The body weight was 7 g, and the forearm length was 37.9 mm. No ectoparasites were observed. On June 5, 2012, the bat was located in a deciduous tree in the forest with two other Natterer’s bats. No transmitter signal was recorded on June 6 and 8, 2012, in an area 1 km from the tree where it was first identified. On June 7, 2012, the Natterer’s bat was located with other bats on another tree 500 m from the first one. On June 9, 2012, the bat was found on a different tree, close to the ground. The same day, the bat specialist removed the radio transmitter from the female bat, as it was clearly ill. The non-aggressive bat showed a weight loss of 2 g in addition to paralysis. The animal died on June 10, 2012. The carcass was stored at −20 °C for one month and then submitted to the laboratory for rabies diagnosis on July 18, 2012.
Rabies diagnosis and molecular biology applied to rabies virus
Routine rabies diagnostic techniques (FAT and RTCIT) were performed as described in “Laboratory Techniques in Rabies” [17]. A necropsy was performed on the Natterer’s bat, and the following organ tissues were aseptically collected: brain, salivary glands, heart, lung, liver, spleen, bladder and kidney. These different organ samples were also tested for the presence of antigen, infectious virus, and viral RNA. Viral RNA was extracted from 200 μL of supernatant from a 10 % (w/v) tissue suspension, using an Iprep™ Pure™ Link Virus Kit according to the manufacturer’s instructions. Extracted RNA was tested by hnRT-PCR [19], Evagreen® RT-qPCR [7] and a rabies TaqMan® assay as described previously [32]. Host RNA controls (18S rRNA and actin) were amplified for each sample with conventional and Evagreen® RT-qPCR. Positive (Duvenhage virus RNA) and negative (RT negative and viral RNA extraction negative) controls were performed in each hnRT-PCR and Evagreen® RT-qPCR run. The limit of detection of the Evagreen® RT-qPCR assay was determined by testing standard DNA diluted serially tenfold. All necessary safety measures were followed to prevent any cross-contamination and false positive results when performing PCRs [13].
Full-genome sequencing
To fully characterize the French Natterer’s bat isolate from 5 μg of total RNA, TRIzol-extracted RNA was obtained from 1 ml homogenate of passaged mouse brain tissue. RNA-Seq library construction was performed at Vertis Biotech (Freising, Germany) after ribo-depletion. Sequencing and assembly were performed by Beckman Coulter Genomics (Danvers, MA and Grenoble, France) using 2 × 100 bp paired-end sequencing on an Illumina HiSeq-2000, generating several tens of millions of reads. Using a proprietary bioinformatics pipeline, reads were recruited at homogeneous coverage using a referenced genome. De novo assembly was performed using the MIRA assembler, yielding a single contig with no need for assembly curation. CDSs were predicted using the Prokov gene predictor with a rabies virus codon usage matrix. CDs were annotated using a proprietary annotation pipeline, using BlastP, against known viral proteins for the selection and transfer of sequences with the best identity match.
Nucleotide sequence analysis and phylogenetics
The nucleotide sequences of the N and G genes were compared to representatives of all lyssavirus species. Phylogenetic trees were constructed using the neighbour-joining (NJ) method with MEGA software, version 5 [29], and using the maximum-likelihood (ML) method in the DNAdist program of the PHYLIP package, version 3.69 [4]. The bootstrap probabilities of each node were calculated using 10,000 replicates for the NJ method and 1,000 replicates for the ML method. Bootstrap values over 70 % indicated significant support for the tree topology.
Similarity plot analysis was performed with SimPlot software (version 3.5.1) [14] using the Kimura 2-parameter model with a window size of 200 nt and a step size of 20 nt.
Results
The results of laboratory testing for antigen, infectious virus, and viral RNA in the different tissue samples tested are presented in Table 1. Antigen, infectious virus, and viral RNA were identified simultaneously in the brain and salivary glands. Figure 2 shows the detection of lyssavirus antigen in the whole-brain impression.
Antigen detection in the whole brain impression of the infected bat. A. Brain impression showing the presence of BBLV antigen. B. FAT negative control (impression of whole bat brain). The FAT was performed as described previously by Dean et al. [2] using a polyclonal anti-rabies-virus conjugate. Total magnification: 400×
The brain was shown to be infected with viral RNA by both conventional hnRT-PCR and Evagreen® RT-qPCR. The results of the rabies TaqMan® assay were negative for the specific detection of RABV, EBLV-1 and EBLV-2. Further analysis of organ samples using Evagreen® RT-qPCR detected traces of viral RNA in all the samples tested, at very low levels (Ct values of ≈ 32.87–33.08), with the exception of the brain and salivary glands, where high levels of RNA were observed (Ct=19.83 and Ct=27.81) (Table 1). The presence of host RNA (18S RNA, β actin) was confirmed in all of the tested samples.
Sequence analysis of the complete genome sequence (11,829 nucleotides) performed with BioEdit 7.0.9.0. software showed 98.7 % identity between the KC169985 isolate and the German BBLV isolate [JF311903].
Sequence analysis of the N (1356 nt) and G (1575 nt) genes showed 98.8 % and 98.3 % identity between the KC169985 isolate and the first BBLV strain [JF311903]. Seventy-six and 81 % nucleotide sequence identity were shown between the Natterer’s bat, EBLV-1 [KC297654] and EBLV-2 [EU293114] N gene sequences, respectively. Analysis of the nucleotide sequence of the N gene showed 75.7 % nucleotide sequence identity between the Natterer’s bat sample from Hémilly and EBLV-1 found in 2012 in Ancy sur Moselle [KC297655], a village 40 km from Hémilly. The phylogenetic relationships between the 12 recognized lyssaviruses, IKOV [JX193798], and the BBLV isolate [JF311903] compared with the French Natterer’s bat sample [KC169985] are represented in Fig. 3.
Phylogenetic relationships between the infected bat and referenced lyssaviruses. Phylogenetic relationships were determined by comparing glycoprotein gene sequences (1575 nucleotides) of the infected bat (KC169985) and 22 referenced lyssavirus strains with MEGA5 software (neighbour-joining method, Tamura Nei model, 10,000 replicates). The GenBank accession numbers as well as the description of the referenced sequences are included for each taxon within the tree
Irrespective of the gene analysed (N or G), phylogenetic analyses performed by the ML and NJ methods produced trees with an identical topology. Whatever the method used or the gene analysed, phylogenetic analysis confirmed that the isolated virus is closely related to BBLV, with a high bootstrap probability of 100 using the ML method and 99 using the NJ method.
Nucleotide sequence identity comparisons using SimPlot analysis based on the complete genome of KC169985 isolate and representative lyssaviruses showed a close relationship between the KC169985 isolate and the first BBLV strain [JF311903] in every genome region (Fig. 4).
Nucleotide similarity plot analysis of the entire genome length of the KC169985 isolate. The complete KC169985 genome sequence was compared with those of three reference lyssaviruses: KHUV [EF614261], EBLV-1 [EU626552] and BBLV [JF311903]. The complete Lyssavirus genomes are drawn to scale. The KC169985 isolate showed greater similarity to BBLV [JF311903] than to KHUV [EF614261] and EBLV-1 [EU626552]
Sequence analysis of the complete genome performed with BioEdit [6] showed that the KC169985 isolate has a higher identity to KHUV (78.4 %), followed by EBLV-2 (77.7 %), ARAV (76.4 %) than to IRKV (73.4 %) and EBLV-1 (73.6 %) (Table 2).
Discussion
Here, we report for the first time the isolation of lyssavirus BBLV from Myotis nattereri in northeastern France. This case was reported in 2010 in the same bat species [5]. This discovery is mainly the result of collaboration between bat workers from the SFEPM and the Anses laboratory within the passive bat rabies surveillance network. As previously suggested by Rupprecht et al. [24], the isolation of BBLV is doubtless due to the increased interest in pathogen discovery and longitudinal studies in wildlife undertaken with bat workers, combined with appropriate diagnostic testing and the molecular biology tools currently used in rabies laboratories to characterise lyssaviruses.
The presence of lyssavirus antigen was shown by Freuling et al. [5] in many parts of the brain of the infected Natterer’s bat in Germany in 2010, while the salivary glands were shown to be negative for the presence of antigen. Unlike previous findings on virus distribution within the organs of this infected Natterer’s bat we found antigen and infectious particles in the salivary glands, with higher levels of viral RNA in the salivary glands and brain than in other tissues. We also detected by RT-qPCR traces of viral RNA in most of the tested organs, including the bladder, lung and kidney. A similar distribution was reported in bats naturally infected with EBLV-2 [9] (salivary glands, tongue, thyroid gland, bladder, heart, lung, intestine and stomach) as well as in RABV-infected bats [1] (salivary glands, tongue, lungs, kidneys, bladder, intestine and faeces). The traces of viral RNA found in organs probably reflect viral dissemination through innervations, most likely occurring in the late stages of disease. Most previous studies indicate that virus transmission between bats occurs through bites from an infected animal to conspecifics within roosts. We detected high levels of viral RNA in the salivary glands of the bat naturally infected with BBLV. Detecting the antigen, virus, and RNA in the salivary glands suggests that these organs contain substantial amounts of infectious virus delivered into the saliva, confirming that bites or scratches by infected animals should be regarded as potential sources of infection among bats.
All SFEPM members participating in the French bat rabies surveillance network are required to be up-to-date in their rabies vaccinations, as laid down in national recommendations delivered by the Ministry of Health in 2005. The three bat workers involved in the discovery were all immunised, with an antibody titre above 4 EU/ml following their last booster injection in early 2012. On July 27 and 30, 2012, they received a post-exposure treatment with a single vaccination. This case underlines the necessity for all bat workers to be immunised against rabies, especially if they handle injured or apparently healthy bats during field investigations.
As reported previously [30, 31], weight loss could be one of the clinical signs of rabies in bats (E. isabellinus) naturally infected with EBLV-1. The same feature was observed in experimentally infected bats (M. daubentonii) using EBLV-2 [10]. Clinical signs in EBLV-1-infected serotine bats often include weakness, inability to fly, and abnormal behaviour, including uncoordinated movement, spasms and, occasionally, paralysis. The Natterer’s bat shown here to be infected with BBLV did not exhibit any of these classical clinical signs of rabies except for paralysis one day before death. The only suspect signs were a weight loss of 2 g associated with a loss of transmitter signal for two days. Usually, the average occupancy of Natterer’s bats in roosts varies from one to four days during the high period of bat activity in summer and two to three weeks during spring and autumn [27]. The inter-roost distance for a colony can reach up to 2 km.
The Natterer’s bat is a non-migratory species (maximum dispersion range, approximately 60 km) and is widespread from the Iberian Peninsula to Caucasia and from Scotland to Scandinavia. The species is present throughout France and in Germany (reviewed in CPEPESC Lorraine [27]), but it is scarce throughout most of its habitat and difficult to catch. The BBLV case reported here raises some questions about BBLV distribution. The first BBLV description reported in Lower Saxony (Germany) was located around 420 km from the forest of Hémilly in France. The distance between the two cases could suggest the presence of this lyssavirus from northeastern France to northern Germany. Despite the relatively high level of rabies surveillance in the two countries, BBLV has not been identified so far. This result can be explained by the fact that only 11 Natterer’s bats out of 2,357 bats were tested for rabies from 2001–2011 throughout France, this species having not been analysed in Moselle (the department where the infected bat was found). The recent discovery of this lyssavirus in France, together with the frequent isolation of EBLV-1 in serotine bats, where an average of three positive cases are reported each year [21], underlines the need to continue reinforcing bat rabies surveillance and raising awareness among professionals involved, including bat workers and veterinary services.
Molecular techniques (the conventional RT-PCR as well as the real-time Evagreen® RT-qPCR) undertaken in complement to the rabies diagnostic techniques (FAT and RTCIT) allowed the detection of Bokeloh bat lyssavirus for the first time in France. Our study showed here that the real-time Evagreen® RT-qPCR performed on the nucleoprotein gene with specific pan-lyssavirus reagents [7] is specific, sensitive and convenient for detecting BBLV in Myotis nattereri.
Whether the forest Natterer’s bat is the principal host for BBLV is unclear. Streicker et al. [28] showed a decrease in the frequency of both cross-species transmission and host shifts with an increase in phylogenetic distance between North American bat species. This raises questions about host/virus evolution in Natterer’s bats with respect to the new species recently identified within the Natterer’s bat population in Europe. Recently, Salicini et al. [25] demonstrated that in the Palearctic region, M. nattereri is a paraphyletic group composed of four clearly differentiated lineages at the species level (M. nattereri, M. escalerai, Myotis sp A and Myotis sp B). M. nattereri is widespread from Central Europe to the Balkans (United Kingdom, France, Germany, Croatia, Montenegro and Serbia), M. escalerai is found across the Iberian peninsula and recently in southern France (eastern Pyrenees), Myotis sp A is found in Italy and the northern part of the Iberian Peninsula, and Myotis sp B is found in the Maghreb [25].
To date, the BBLV strain has never been found in Europe except in 2010 in Germany, in a single animal. Field studies are necessary to investigate BBLV circulation among forest bats, in close collaboration with bat workers.
Abbreviations
- BBLV:
-
Bokeloh bat lyssavirus
- Ct:
-
Threshold cycle
- EBLV-1:
-
European bat lyssavirus type 1
- EBLV-2:
-
European bat lyssavirus type 2
- FAT:
-
Fluorescent antibody test
- G:
-
Glycoprotein
- L:
-
Polymerase
- M:
-
Matrix protein
- N:
-
Nucleoprotein
- nt:
-
Nucleotides
- P:
-
Phosphoprotein
- RTCIT:
-
Rabies tissue culture infection test
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- qPCR:
-
Quantitative real-time polymerase chain reaction
References
Allendorf SD, Cortez A, Heinemann MB, Harary CM, Antunes JM, Peres MG, Vicente AF, Sodre MM, da Rosa AR, Megid J (2012) Rabies virus distribution in tissues and molecular characterization of strains from naturally infected non-hematophagous bats. Virus Res 165:119–125
Dean D, Abelseth M, Atanasiu P (1996) The fluorescent antibody test. In: Meslin F, Kaplan M, Koprowski H (eds) Laboratory techniques in rabies, 4th edn. World Health Organization, Geneva, pp 88–95
Dietz C, Von Helversen O (2004) Illustrated identification key to the bats of Europe. http://public.carnet.hr/speleo/znanost/sismisi/Dietz_von_Helversen_2004IDkey_2.pdf
Felsenstein J (1989) PHYLIP—phylogeny inference package (Version 3.2). Cladistics 5:164–166
Freuling CM, Beer M, Conraths FJ, Finke S, Hoffmann B, Keller B, Kliemt J, Mettenleiter TC, Muhlbach E, Teifke JP, Wohlsein P, Muller T (2011) Novel lyssavirus in Natterer’s bat, Germany. Emerg Infect Dis 17:1519–1522
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis. http://www.mbio.ncsu.edu/BioEdit/bioedit.html
Hayman DTS, Banyard AC, Wakeley PR, Harkess G, Marston D, Wood JLN, Cunningham AA, Fooks AR (2011) A universal real-time assay for the detection of Lyssaviruses. J Virol Methods 177:87–93
ICTV (2011) ICTV official taxonomy: updates since the 8th report. Vertebrate
Johnson N, Wakeley PR, Brookes SM, Fooks AR (2006) European bat lyssavirus type 2 RNA in Myotis daubentonii. Emerg Infect Dis 12:1142–1144
Johnson N, Vos A, Neubert L, Freuling C, Mansfield KL, Kaipf I, Denzinger A, Hicks D, Nunez A, Franka R, Rupprecht CE, Muller T, Fooks AR (2008) Experimental study of European bat lyssavirus type-2 infection in Daubenton’s bats (Myotis daubentonii). J Gen Virol 89:2662–2672
Kuzmin IV, Hughes GJ, Botvinkin AD, Orciari LA, Rupprecht CE (2005) Phylogenetic relationships of Irkut and West Caucasian bat viruses within the Lyssavirus genus and suggested quantitative criteria based on the N gene sequence for lyssavirus genotype definition. Virus Res 111:28–43
Kuzmin IV, Bozick B, Guagliardo SA, Kunkel R, Shak JR, Tong S, Rupprecht CE (2011) Bats, emerging infectious diseases, and the rabies paradigm revisited. Emerg Heath Threats J 4:1–13
Kwok S, Higuchi R (1989) Avoiding false positives with PCR. Nature 339:237–238
Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, Ingersoll R, Sheppard HW, Ray SC (1999) Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol 73:152–160
Marston DA, Horton DL, Ngeleja C, Hampson K, McElhinney LM, Banyard AC, Haydon D, Cleaveland S, Rupprecht CE, Bigambo M, Fooks AR, Lembo T (2012) Ikoma lyssavirus, highly divergent novel lyssavirus in an African civet. Emerg Infect Dis 18:664–667
McElhinney LM, Marston DA, Leech S, Freuling CM, van der Poel WHM, Echevarria J, Vázquez-Moron S, Horton DL, Müller T, Fooks AR (2013) Molecular epidemiology of bat lyssaviruses in Europe. Zoon Publ Health 60:35–45
Meslin FX, Kaplan MM, Koprowski H (1996) Laboratory techniques in rabies, 4th edn. World Health Organization, Geneva
Mohr W (1957) Rabies. Med Klin 52:1057–1060
Picard-Meyer E, Bruyere V, Barrat J, Tissot E, Barrat MJ, Cliquet F (2004) Development of a hemi-nested RT-PCR method for the specific determination of European Bat Lyssavirus 1. Comparison with other rabies diagnostic methods. Vaccine 22:1921–1929
Picard-Meyer E, Barrat J, Tissot E, Verdot A, Patron C, Barrat MJ, Cliquet F (2006) Bat rabies surveillance in France, from 1989 through May 2005. Dev Biol 125:283–288
Picard-Meyer E, Cliquet F (2011) Programme d’épidémiosurveillance des infections à lyssavirus chez les chiroptères. Résultats et analyses du 1er janvier au 31 décembre 2010. http://www.cpepesc.org/IMG/pdf/bulletin_epidemioS_chauves-souris_2010_version2.pdf. Rapport Ansès - Laboratoire de la rage et de la faune sauvage de Nancy. Ansès - Laboratoire de la rage et de la faune sauvage de Nancy, Malzéville, pp 1–20
Picard-Meyer E, Dubourg-Savage MJ, Arthur L, Barataud M, Becu D, Bracco S, Borel C, Larcher G, Meme-Lafond B, Moinet M, Robardet E, Wasniewski M, Cliquet F (2011) Active surveillance of bat rabies in France: a 5-year study (2004–2009). Vet Microbiol 151:390–395
Picard-Meyer E, Borel C, Moinet M, Servat A, Rasquin P, Cliquet F (2012) Découverte d’un Vespertilion de Natterer infecté par le Lyssavirus BBLV en Moselle en 2012. Bull Epidémiol Santé Anim- Alimentation, p 25
Rupprecht CE, Turmelle A, Kuzmin IV (2011) A perspective on lyssavirus emergence and perpetuation. Curr Opin Virol 1:662–670
Salicini I, Ibanez C, Juste J (2011) Multilocus phylogeny and species delimitation within the Natterer’s bat species complex in the Western Palearctic. Mol Phylogenet Evol 61:888–898
Schatz J, Fooks AR, McElhinney L, Horton D, Echevarria J, Vázquez-Moron S, Kooi EA, Rasmussen TB, Müller T, Freuling CM (2013) Bat rabies surveillance in Europe. Zoon Public Health 60:22–34
Schwaab F, Knochel A, Jouan D (2009) Connaître et protéger les chauves-souris de Lorraine. Ciconia
Streicker DG, Turmelle AS, Vonhof MJ, Kuzmin IV, McCracken GF, Rupprecht CE (2010) Host phylogeny constrains cross-species emergence and establishment of rabies virus in bats. Science 329:676–679
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Vazquez-Moron S, Juste J, Ibanez C, Aznar C, Ruiz-Villamor E, Echevarria JE (2008) Asymptomatic rhabdovirus infection in meridional serotine bats (Eptesicus isabellinus) from Spain. Dev Biol 131:311–316
Vazquez-Moron S, Juste J, Ibanez C, Ruiz-Villamor E, Avellon A, Vera M, Echevarria JE (2008) Endemic circulation of European bat lyssavirus type 1 in serotine bats, Spain. Emerg Infect Dis 14:1263–1266
Wakeley PR, Johnson N, McElhinney LM, Marston D, Sawyer J, Fooks AR (2005) Development of a real-time, TaqMan reverse transcription-PCR assay for detection and differentiation of lyssavirus genotypes 1, 5, and 6. J Clin Microbiol 43:2786–2792
Webster WA, Casey GA (1996) Virus isolation in neuroblastoma cell culture. In: Meslin FX, Kaplan MM, Koprowski H (eds) Laboratory techniques in rabies, 4th edn. World Health Organization, Geneva, pp 96–103
Acknowledgments
We are grateful to the local bat conservation association members (CPEPESC Lorraine) for their full collaboration in the bat rabies surveillance network and for their support, especially D. Jouan. We are also grateful to the French National Forestry Office, ONF. We would like to thank M. J. Duchêne for excellent technical support, along with the two teams focusing on rabies diagnosis (V. Brogat, E. Litaize) and molecular biology (M. Biarnais, J. L. Schereffer). The inventory of bats, which led to the discovery of the infected Natterer’s bat was funded by DREAL Lorraine. This research was funded by Anses, the French Agency for Food, Environmental and Occupational Health & Safety.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Picard-Meyer, E., Servat, A., Robardet, E. et al. Isolation of Bokeloh bat lyssavirus in Myotis nattereri in France. Arch Virol 158, 2333–2340 (2013). https://doi.org/10.1007/s00705-013-1747-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-013-1747-y