Abstract
A replication-defective, recombinant Sindbis virus vector was utilized in a novel immunization strategy to induce humoral and cellular responses against hepatitis C virus (HCV). The recombinant vector, pVaXJ-E1E2, expressing the gene for HCV glycoproteins E2 and E1, was constructed by inserting the E1E2 gene into the replicon pVaXJ, a DNA vector derived from Sindbis-like virus XJ-160. The defective replicon particles, XJ-E1E2, were produced by transfecting BHK-21E+Capsid cells, the packaging cell lines for the vector from XJ-160 virus, with pVaXJ-E1E2. Both glycoproteins, E2 and E1, were stably expressed, as indicated by immunofluorescence assay (IFA) and Western blotting. Mice were vaccinated using a prime-boost strategy with XJ-E1E2 particles combined with Freund’s incomplete adjuvant via intramuscular injection at 0 and 2 weeks. HCV-specific IgG antibody levels and cellular immune responses were evaluated by IFA and IFN-γ ELISPOT, respectively. The results showed that the defective XJ-E1E2 particles in combination with Freund’s incomplete adjuvant induced effective humoral and cellular immune responses against HCV glycoprotein E1 or E2, suggesting that a defective Sindbis particle vaccine is capable of eliciting an effective immune response. These findings have important implications for the development of HCV vaccine candidates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Persistent hepatitis C virus (HCV) infection is a major health problem worldwide. It affects ~3 % of the world’s population and is an important contributor to chronic liver disease [1]. The standard combination treatment for chronic hepatitis C, namely, interferon (IFN)-α and ribavirin, is curative in less than one-half of all HCV cases [2, 3]. In 2011, two inhibitors of the virally encoded NS3/4 protease have become part of standard therapy, which has improved treatment rates but can exacerbate the problematic side effects [4]. Therefore, there is an urgent need for alternative therapies and effective prophylactic vaccines. The fact that one of four HCV-infected individuals spontaneously clears the HCV infection provides hope for the successful development of an effective vaccine. Development of effective prophylactic and therapeutic vaccines against HCV is of high priority because it could significantly lower the chronicity rate and thus have a major influence on the disease burden. Considerable effort has been directed toward the development of a safe and effective HCV vaccine, but it remains a major challenge because of the high variability of HCV.
HCV, a member of the family Flaviviridae, has a positive-sense RNA genome that encodes a polyprotein, which is cleaved by cellular and viral proteases into structural and non-structural proteins. The glycoproteins E1 and E2, which exhibit a high degree of variability, are responsible for cell attachment and virus entry. The site of greatest variability is within the E2 envelope glycoprotein (hypervariable region 1), a major target of neutralizing antibodies [5]. The general feature of most vaccines is induction of neutralizing antibodies. In addition, HCV persistence is associated with a weak and dysfunctional virus-specific T cell response [6, 7], and strong HCV-specific cellular immune responses are likely important in viral clearance and possibly protection from HCV infection [7–10]. Therefore, the goal of any HCV vaccine strategy is to engineer a candidate vaccine to elicit an efficient adaptive immune response, in particular a cell-mediated response.
Defective alphaviral replicon vectors have been shown in several animal models to induce robust cellular, humoral, and mucosal immune responses that are specific for the replicon-expressed antigen [11–13]. In particular, elicitation of broad CD4+ and CD8+ T cell responses to HCV E1E2 and NS345 as well as anti-E1E2 cross-neutralizing antibodies can be obtained by vaccination with chimeric, defective alphaviruses derived from Sindbis virus and Venezuelan equine encephalitis virus that express these HCV genes [14]. In this study, based on the DNA vector pVaXJ from Sindbis-like virus XJ-160, we have constructed replication-defective HCV particles to induce humoral and cellular responses against the HCV glycoproteins E2 and E1, confirming the idea that an alphavirus-based HCV vaccine is capable of inducing effective immune responses and demonstrating that this is a promising vaccination strategy against HCV infection.
Materials and methods
Cell line, plasmids, and antibodies
The use of BHK-21E+Capsid cells, the packaging cell line (PCL) for the vector from XJ-160 virus, simplifies large-scale vector production and facilitates broad application of alphaviruses [15]. The BHK-21E+Capsid cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (FBS) and 100 U/mL each of penicillin and streptomycin. The DNA-based vector pVaXJ was constructed by placing the recombinant genome of Sindbis-like virus XJ-160 under the control of the human cytomegalovirus (CMV) promoter of the plasmid pVAX1, in which the viral structural genes were replaced by a polylinker cassette to allow the insertion of heterologous genes [16]. The plasmid pVRC-HCV, encoding the HCV E1E2 gene, and the mouse monoclonal antibodies (mAbs) to the HCV E1 and E2 proteins were kindly provided by Dr. Tan (China CDC). HCV-positive human serum was kindly provided by Dr. Lu (China CDC).
Generation of replication-defective particles
The HCV E1E2 gene (amino acids [aa] 170–746 from HCV 1b subtype, Hebei isolate [17], NCBI accession no. L02836) was amplified from the plasmid pVRC-HCV. The recombinant construct pVaXJ-E1E2 was made by cloning the HCV E1E2 gene into the DNA-based vector pVaXJ using unique sites for the restriction enzymes FseI and AscI. The recombinant construct was confirmed by sequence analysis. Replicon particles were generated by transfection of BHK-21E+Capsid cells with pVaXJ-E1E2. Replicon particles expressing HCV E1E2 were harvested as culture supernatants 48 h post-transfection, clarified by filtration, and purified by cation-exchange chromatography. Replicon particle titers, reported in infectious units (IU) per milliliter, were determined by intracellular staining for the expressed HCV proteins (E1 and E2) following overnight infection of BHK-21E+Capsid cells, as described previously [13].
Characterization of the replicon particles
Target protein expression after treatment with replicon particles was detected by immunofluorescence assay (IFA) and western blotting (WB). IFA tests were performed as described previously [18]. In brief, the BHK-21E+Capsid cells were harvested 48 h post-transfection with pVaXJ-E1E2 and fixed in cold acetone. After open-air drying and a second wash with cold phosphate-buffered saline (PBS), 1:50 diluted HCV-positive serum was added and incubated at 37 °C for 30 min in a moist chamber. After washing three times with PBS and air-drying, fluorescein-isothiocyanate (FITC)-labeled goat anti-human antibody (Sigma) diluted 1:100 with azovan blue was added. Finally, the slides were observed under a fluorescence microscope. The BHK-21E+Capsid cells transfected with pVaXJ-E1E2 were collected and lysed 48 h later. Lysates were then separated on a 10 % or 15 % polyacrylamide gel and transferred by electroblotting to a polyvinylidene fluoride membrane. The membrane was blocked with 5 % skim milk for 1 h at 37 °C and then incubated with mAb against the HCV E1 or E2 protein overnight at 4 °C. After being washed three times with PBS containing 0.5 % Tween-20, the membrane was protected from light and incubated with goat anti-mouse antibody (Sigma) for 1 h at 37 °C. After washing three times in PBS, bands were detected using an infrared imaging system. In addition, BHK-21E+Capsid cells transfected with pVaXJ-E1E2 in T25 flasks were fixed for transmission electron microscopy with Ito solution as described previously [19]. Ultrathin sections were cut on a Reichert-Leica Ultracut S ultramicrotome, stained with lead citrate and examined using a Philips 201 or CM-100 electron microscope at 60 kV. Negative-stain electron microscopy was performed on virions purified from a clarified culture supernatant from the transfected cells as described previously [20].
Animal experiments
To study the humoral and cellular responses against HCV, two groups of female BALB/c mice were immunized with replicon particles at 6–8 weeks of age. In brief, 12 female BALB/c mice from the experimental group were injected intramuscularly in the tibialis anterior muscle at weeks 0 and 2. We used 5 × 106 IU of replication-defective particles mixed with Freund’s incomplete adjuvant in the prime-boost studies. In addition, mice injected with PBS were used as a control group. All of the mice were bled at weekly intervals from 0 to 8 weeks after they were immunized. The mice were sacrificed at weeks 2, 4, and 8, and their spleens were harvested for testing in T cell response assays.
Analysis of the immune response in vaccinated mice
The immune response of the vaccinated mice was then examined, and enzyme-linked immunospot (ELISPOT) assays and IFA were performed to determine the levels of cellular immune response (CIR) and humoral immune response (HIR), respectively. To determine the levels of HCV-specific IgG antibody, IFA was performed as follows: The sera were heat-inactivated at 56 °C for 30 min and then serially diluted twofold. The diluted sera were added to the wells of slides containing replicon particle antigen. After incubation at 37 °C for 60 min, the slides were washed four times with PBS and air-dried. FITC-labeled goat anti-mouse IgG antibody (Sigma) diluted with Evan’s blue solution (0.02 %) was added to each well and incubated at 37 °C for 60 min. The slides were washed again with PBS and observed under a fluorescence microscope. For the ELISPOT assay, a peptide library of HCV structural proteins (E1/E2), based on the Hebei isolate sequence of HCV genotype 1b, was synthesized at lengths of 13–17 aa with an overlap of 10 aa between fragments. The E1 and E2 peptide pools contained 42 and 79 peptides, respectively. ELISPOT assays were performed as described previously [21]. The CIR level was determined by the number of IFN-γ-positive spot-forming cells (SFCs) against the E1 or E2 peptide pools per million mononuclear spleen cells (MNCs). To analyze CIR further, interleukin-6 (IL-6) and IL-10 levels in spleen cell culture supernatants were detected as described previously [14].
Data analysis
Significant differences between the experimental and control groups were evaluated using the one-way ANOVA analysis function in the SPSS software package (release 12.1; SPSS Inc., Chicago, IL, USA). Differences were considered significant if p < 0.05.
Ethical approval
According to the medical research regulations of the Ministry of Health, China, this study was approved by the ethics committee of China CDC, which uses international guidelines to ensure confidentiality, anonymity, and informed consent.
Results
Construction and expression of replication-defective HCV particles
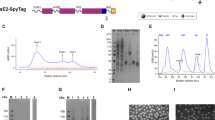
We constructed a replication-defective recombinant vector, pVaXJ-E1E2, by cloning the HCV E1E2 gene (170–746 aa from HCV 1b subtype, Hebei isolate) into the pVaXJ vector. Replicon particles expressing HCV E1E2 (XJ-E1E2) were produced by transfecting BHK-21E+Capsid cells with pVaXJ-E1E2. Expression of the target proteins was confirmed by IFA and WB. BHK-21E+Capsid cells transfected with pVaXJ-E1E2 were assayed for HCV-specific protein expression 48 h postinfection. Compared to negative controls (Fig. 1A), the pVaXJ-E1E2-transfected cells yielded positive immunofluorescence staining (Fig. 1B), indicating that HCV-specific protein was expressed in BHK-21E+Capsid cells. Western blotting (Fig. 1C) using mouse mAbs against HCV E1 or E2 protein further confirmed that both proteins were expressed in pVaXJ-E1E2-transfected cells, and negative-stain electron microscopy indicated that replicon particles purified from the supernatants of transfected BHK-21E+Capsid cells were spheres with a diameter of 60 to 80 nm (Fig. 2A). Transmission electron microscopy revealed replicon particles in the cytoplasm, which were observed inside vacuoles, presumably in the Golgi apparatus (Fig. 2B).
Expression of HCV target proteins was confirmed by immunofluorescence assay (IFA) (A and B) and western blotting (WB) (C). Mock-transfected BHK-21E+Capsid (control) cells (A) and BHK-21E+Capsid cells (B) transfected with the recombinant plasmid pVaXJ-E1E2 were fixed 48 h after infection and stained with a human serum positive for antibodies against HCV. In the WB experiment (C), the lysates of control and pVaXJ-E1E2-transfected BHK-21E+Capsid cells were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Blots were probed with mAbs directed against the HCV E1 and E2 glycoproteins, respectively. Lanes M and C represent the protein marker and control, respectively
XJ-E1E2 vaccination-induced CIR in mice
To detect the cellular immune response elicited by replicon particles (XJ-E1E2), splenocytes were harvested 2, 4, and 8 weeks after immunization and stimulated with an HCV peptide pool representing E1 or E2 protein. As shown in Fig. 2A, IFN-γ-positive SFCs specific for both E1 and E2 peptide pools were detected in the experiment group, while almost no spots were found in the control group. The experimental group injected with PBS had significantly fewer SFCs than did the group that received replicon particles (p < 0.05), and there were no significant differences in CIRs against E1 versus E2 peptide pools (Fig. 3A). Compared to the control group, XJ-E1E2 particles produced HCV-specific IL-6 and IL-10 after in vitro stimulation with the E1 or E2 peptide pool (Fig. 3B, C). These results indicate that replication-defective XJ-E1E2 particles mixed with Freund’s incomplete adjuvant induced HCV-specific cytokine production from CD8+ T cells (IFN-γ) and CD4+ T cells (IL-6 and IL-10).
Cellular immune response (CIR) against HCV antigens (E1 or E2) induced by XJ-E1E2 virus-like particles. (A) An ELISPOT assay of splenocytes. Each point represents the average number of IFN-γ-positive SFCs per million MNCs from three different mice, plus the standard deviation. (B and C) IL-6 and IL-10 levels in spleen cell culture supernatants. The data shown are representative of three independent experiments
HIR in mice detected by IFA
To assess the humoral immune response in mice vaccinated with XJ-E1E2, the levels of IgG antibody against HCV was monitored by IFA. An effective HIR against HCV was detected within 8 weeks after the mice were inoculated with XJ-E1E2 particles, and the IFA titer of HCV-specific IgG antibodies reached its peak of 1:213 at 4 weeks (the second week after the boost immunization; Fig. 4). The IgG titer at 2 weeks was significantly lower than that at 3 weeks (the first week after the boost immunization; p < 0.05). Forty-eight days after the mice were inoculated with the particles, the IFA titer of HCV-specific IgG also achieved a similar level to that of the second week (the first week after prime vaccination). These results suggest that replicon particles representing HCV E1E2 proteins could induce an effective HIR and that the boost immunization played an important role in promoting high-level IgG antibody production.
Discussion
A number of approaches, including the use of alphavirus-like particles and defective or attenuated viral vectors with or without a prime-boost strategy, have been used to generate T cell responses against HCV antigens [11–13]. E1E2 particles can be assembled when expressed in insect cells or the lentivirus expression system [22, 23]. The use of virus-like particles pseudotyped with E2 and/or E1 HCV envelope glycoproteins has proven successful for inducing high-titer anti-E2 and/or anti-E1 antibodies as well as neutralizing antibodies in both mice and macaques [24]. In addition, a meta-analysis of HCV vaccine efficacy in chimpanzees has indicated the importance of structural proteins that may activate T cell responses and thus mediate viral clearance [25]. Therefore, we chose the structural gene E1E2 of the HCV isolates dominant in China (1b) to develop HCV replicon particles with the DNA-based vector pVaXJ. IFA and western blot analyses indicated that HCV-specific proteins and the E1 and E2 proteins were expressed efficiently in cells infected with pVaXJ-E1E2.
Because HCV is very heterogeneous, an ideal HCV vaccine needs to elicit protective, broadly cross-neutralizing antibodies as well as widely reactive cellular immunogenicity. Previous work has shown that a single immunization, priming with adjuvanted envelope glycoproteins and non-structural proteins of HCV followed by boosting with defective alphavirus particles expressing those genes, was optimal for eliciting the array of known protective immune responses against HCV infection [14]. The present work shows that replication-defective XJ-E1E2 particles mixed with Freund’s incomplete adjuvant could also elicit all of the immune responses known to be associated with protection from HCV infection, including neutralizing antibodies (Fig. 4) as well as broad cellular immune responses against HCV (Fig. 3). These results confirm that defective HCV vaccines based on alphavirus particles may have potential therapeutic use and/or prophylactic efficacy.
Freund’s adjuvants are irreplaceable components for inducing immune responses in many experimental animal models. Generally, it is assumed that incomplete and complete Freund’s adjuvant act by prolonging the lifetime of injected antigen, by enhancing its effective delivery to the immune system, and by providing a complex set of signals to the innate compartment of the immune system, resulting in altered leukocyte proliferation and differentiation. Compared to complete Freund’s adjuvant, incomplete Freund’s adjuvant has been the more commonly used immune-stimulating adjuvant for experimental models concerned with HCV vaccination [26–28]. Therefore, we chose incomplete Freund’s adjuvant as the adjuvant together with our replicon particles to vaccinate the mice in this study.
An effective vaccine and new therapeutic methods for HCV are needed, and potential HCV vaccines should induce robust humoral and cellular immune responses. Previous research has indicated that defective alphaviruses expressing HCV glycoprotein genes may have the ability to elicit strong B and T cell immune responses against target antigens [14]. Our data provide further support for the idea that an alphavirus-based HCV vaccine has promise against HCV infection and is capable of inducing dual-sided immune responses. However, information regarding the types of immune responses related to protection or clearance of HCV after vaccination is still lacking. Future studies should also focus on higher-level immunological analyses of the multifunctional activities of T cells and T-cell phenotypes.
Reference
Shepard CW, Finelli L, Alter MJ (2005) Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 5:558–567
Arribillaga L, Sarobe P, Arina A, Gorraiz M, Borras-Cuesta F, Ruiz J, Prieto J, Chen L, Melero I, Lasarte JJ (2005) Enhancement of CD4 and CD8 immunity by anti-CD137 (4–1BB) monoclonal antibodies during hepatitis C vaccination with recombinant adenovirus. Vaccine 23:3493–3499
Capone S, Meola A, Ercole BB, Vitelli A, Pezzanera M, Ruggeri L, Davies ME, Tafi R, Santini C, Luzzago A, Fu TM, Bett A, Colloca S, Cortese R, Nicosia A, Folgori A (2006) A novel adenovirus type 6 (Ad6)-based hepatitis C virus vector that overcomes preexisting anti-Ad5 immunity and induces potent and broad cellular immune responses in rhesus macaques. J Virol 80:1688–1699
Ploss A, Dubuisson J (2012) New advances in the molecular biology of hepatitis C virus infection: towards the identification of new treatment targets. Gut 61:i25–i35
Simmonds P (2004) Genetic diversity and evolution of hepatitis C virus-15 years on. J Gen Virol 85:3173–3188
Folgori A, Capone S, Ruggeri L, Meola A, Sporeno E, Ercole BB, Pezzanera B, Tafi R, Arcuri M, Fattori E, Lahm A, Luzzago A, Vitelli A, Colloca S, Cortese R, Nicosia A (2006) A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med 12:190–197
Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM (2003) HCV persistence and immune evasion in the absence of memory T cell help. Science 302:659–662
Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM (2003) Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med 197:1645–1655
Shiina M, Rehermann B (2006) Hepatitis C vaccines: inducing and challenging memory T cells. Hepatology 43:1395–1398
Thimme R, Neumann-Haefelin C, Boettler T, Blum HE (2008) Adaptive immune responses to hepatitis C virus: from viral immunobiology to a vaccine. Biol Chem 389:457–467
Kirman JR, Turon T, Su H, Li A, Kraus C, Polo JM, Belisle J, Morris S, Seder RA (2003) Enhanced immunogenicity to Mycobacterium tuberculosis by vaccination with an alphavirus plasmid replicon expressing antigen 85A. Infect Immun 71:575–579
Polo JM, Gardner JP, Ji Y, Belli BA, Driver DA, Sherrill S, Perri S, Liu MA, Dubensky TW Jr (2000) Alphavirus DNA and particle replicons for vaccines and gene therapy. Dev Biol (Basel) 104:181–185
Perri S, Greer CE, Thudium K, Doe B, Legg H, Liu H, Romero RE, Tang Z, Bin Q, Dubensky TW, JrM Vajdy Jr, Otten GR, Polo JM (2003) An alphavirus replicon particle chimera derived from Venezuelan equine encephalitis and Sindbis viruses is a potent gene-based vaccine delivery vector. J Virol 77:10394–10403
Lin YL, Kwon T, Polo J, Zhu YF, Coates S, Crawford K, Dong C, Wininger M, Hall J, Selby M, Coit D, Medina-Selby A, McCoin C, Ng P, Drane D, Chien D, Han J, Vajdy M, Houghton M (2008) Induction of broad CD4+ and CD8+ T-Cell responses and cross-neutralizing antibodies against Hepatitis C virus by vaccination with Th1-adjuvanted polypeptides followed by defective alphaviral particles expressing envelope glycoproteins gpE1 and gpE2 and nonstructural proteins 3, 4, and 5. J Viro 82:7492–7503
Zhu WY, Liang GD (2009) Selection and characterization of packaging cell lines for XJ-160 virus. Intervirology 52:100–104
Zhu WY, Li JJ, Tang L, Wang HQ, Li J, Fu JJ, Liang GD (2011) Glycoprotein is enough for sindbis virus-derived DNA vector to express heterogenous genes. Virol J 8:344
Bi SL, Bai XH, Cong ME, Liu CB (1993) Sequence analysis and variation in hepatitis C virus isolate from Hebei Province, China. Chin J Virol 9:114–127
Zhu WY, Yang YL, Fu SH, Wang LH, Zhai YG, Tang Q, Liang GD (2009) Substitutions of 169Lys and 173Thr in nonstructural protein 1 influence the infectivity and pathogenicity of XJ-160 virus. Arch Virol 154:245–253
Popov VL, Chen SM, Feng HM, Walker DH (1995) Ultrastructural variation of cultured Ehrlichia chaffeensis. J Med Microbiol 43:411–421
Li C, Liu F, Liang M (2010) Hantaviruslike particles generated in CHO cells induce specific immune responses in C57BL/6 mice. Vaccine 28:4294–4300
Guan J, Wen B, Deng Y, Zhang K, Chen H, Wu XB, Ruan L, Tan WJ (2011) Effect of route of delivery on heterologous protection against HCV induced by an adenovirus vector carrying HCV structural genes. Virol J 8:56
Elmowalid GA, Qiao M, Jeong SH, Jeong SH, Borg BB, Baumert TF, Sapp RK, Hu Z, Muethy K, Liang TJ (2007) Immunization with hepatitis C virus-like particles results in control of hepatitis C virus infection in chimpanzees. Proc Natl Acad Sci USA 104:8427–8432
Cocquerel L, Kuo CC, Dubuisson J, Levy S (2003) CD81-dependent binding of hepatitis C virus E1E2 heterodimers. J Virol 77:10677–10683
Garrone P, Fluckiger AC, Mangeot PE, Gauthier E, Dupeyrot-Lacas P, Mancip J, Cangialosi A, Chéné ID, LeGrand R, Mangeot I, Lavillette D, Bellier B, Cosset FL, Tangy F, Klatzmann D, Dalba C (2011) A prime-boost strategy using virus-like particles pseudotyped for HCV proteins triggers broadly neutralizing antibodies in macaques. Sci Transl Med 3: 94ra71
Dahari H, Feinstone SM, Major ME (2010) Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins. Gastroenterol 139:965–974
Nakata S, Estes MK, Chiba S (1988) Detection of human calicivirus antigen and antibody by enzyme-linked immunosorbent assays. J Clin Microbiol 26:2001–2005
Sabo MC, Luca VC, Prentoe J, Hopcraft SE, Blight KJ, Yi MK, Lemon SM, Ball JK, Bukh J, Evans MJ, Fremont DH, Diamond MS (2011) Neutralizing monoclonal antibodies against hepatitis C virus E2 protein bind discontinuous epitopes and inhibit infection at a postattachment step. J Virol 85:7005–7019
Mecchia M, Casato M, Tafi R, Filocamo G, Bonomo L, Fiorilli M, Cortese R, Migliaccio G, Nicosia A (1996) Nonrheumatoid IgM in human hepatitis C virus-associated type II cryoglobulinemia recognize mimotopes of the CD4-like LAG-3 protein. J Immunol 157:3727–3736
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81160353; 30970160) and the China Mega-Project for Infectious Disease (2011ZX10004-001), and a Development Grant of State Key Laboratory for Infectious Disease Prevention and Control (2011SKLID205).
Author information
Authors and Affiliations
Corresponding authors
Additional information
W. Zhu, J. Fu, J. Lu and Y. Deng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhu, W., Fu, J., Lu, J. et al. Induction of humoral and cellular immune responses against hepatitis C virus by vaccination with replicon particles derived from Sindbis-like virus XJ-160. Arch Virol 158, 1013–1019 (2013). https://doi.org/10.1007/s00705-012-1564-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-012-1564-8