Abstract
Antibodies to two crucial regions, the third variable loop (V3) of gp120 and the membrane-proximal external region (MPER) of gp41 are important for HIV-1 neutralization. We here evaluated the relative binding of polyclonal plasma antibodies from 99 HIV-1-infected individuals from India to the consensus–C V3 and MPER peptides and observed immunodominance of V3 over MPER (p < 0.0001). We further examined the V3- and MPER-specific antibody correlates with clinical parameters. Our results revealed that anti-V3 antibody titers are significantly lower in patients on ART compared to drug-naive individuals (p < 0.0001), most likely due to a decrease in plasma viral load, irrespective of their CD4 counts and total IgG. No such association was observed for MPER, with a similar trend in four follow-up patients. These findings strongly suggest that high titers of V3-specific antibodies are dependent on persistence of virus in circulation, while antibodies to MPER are probably not.
Similar content being viewed by others
Introduction
Induction of antibodies is undoubtedly an important feature of a prophylactic vaccine. During the natural course of human immunodeficiency virus type 1 (HIV-1) infection, a repertoire of virus-specific antibodies are generated against essentially all HIV-1 proteins, but only the antibodies directed to envelope glycoprotein gp160 are capable of neutralizing viruses [1, 2]. The present knowledge about HIV-1 immunology has accumulated through studies of polyclonal and monoclonal antibodies from HIV-1-infected individuals [3–7]. It is encouraging that humans produce antibodies that neutralize HIV-1 [8]; however, the underlying mechanism remains poorly understood. HIV-1 diversity is one of the major obstacles for HIV-1 vaccine development [9, 10], adding complexity to the type of immune responses evoked during infection [11]. With the expansion of HIV-1 to 13 subtypes or sub-subtypes and more than 40 circulating recombinant forms [12], it has become extremely difficult to evolve strategies to combat the virus [13, 14]. HIV-1 subtype C accounts for the majority of the global infections but is less studied than the other subtypes, and in India, more than 90% of individuals with HIV-1 are reported to be infected with clade-C viruses [15].
The viral envelope glycoprotein gp160 (gp120 and gp41) is the first virus-associated protein to encounter the immune system, and it is involved in a series of early events leading to conformational changes in its structure that are necessary for successful entry of the virus into host cells [16, 17]. This process is initiated by interaction of viral gp120 with the primary receptor (CD4) and the coreceptor (CXCR4/CCR5) [18–20] and, eventually, gp41-mediated fusion of the viral membrane with the host-cell membrane [21]. Therefore, the non-covalently associated gp120 and gp41 envelope proteins [22] are considered potential targets for virus neutralizing antibodies (NtAbs) [23]. The third variable region (V3) of gp120 and the membrane-proximal external region (MPER) of gp41 are the most extensively studied regions in HIV-1. V3 is highly immunodominant, and plasma from essentially all HIV-1-infected individuals has high titers of anti-V3 antibodies [24]. Despite its sequence variability, V3 has structurally conserved motifs that are targets of broadly neutralizing monoclonal antibodies (bNtAbs) [25, 26]. The MPER comprises the last 24 C-terminal amino acids of the gp41 ectodomain, is highly conserved [27] and, importantly, is recognized by three HIV-1 bNtAbs [28–30]. Structural conservation and the presence of epitopes recognized by bNt MAbs are the major attributes that make V3 and MPER attractive targets for vaccine design.
Although both V3 and MPER have been shown to be important for neutralization, they differ greatly in the extent of their immunogenicity. HIV-1-infected individuals have shown high titers of anti-V3 antibodies [31]. In addition, antibodies directed to epitopes within the V3 and MPER have been associated with disease progression and other clinical factors [32, 33]. The HIV-1-specific humoral response has not yet been assessed in detail in Indian HIV-1-infected patients. Here, we evaluate the relative reactivity of polyclonal plasma antibodies from 99 HIV-1-infected North Indians that were specific for V3 and MPER. We observed high titres of V3 antibodies compared to anti-MPER antibodies. The patients were assumed to be infected with HIV-1 subtype C, which is the predominant subtype in India [34]. Indeed, the envelope sequence of a few samples confirmed these to be subtype C (data not shown). We further assessed the V3- and MPER-specific antibody correlates with clinical parameters (drug status, total IgG levels, CD4 count, and plasma viral load) in order to see whether any of these parameters account for the difference in immunogenicity between these two regions. This study suggests that V3 is highly immunodominant compared to MPER, irrespective of clinical and immunological factors, and that the anti-V3 antibody titers correlate with plasma viremia, while those of MPER remain unaltered. This is the first report on characterization of V3- and MPER-specific immune correlates and their association with clinical variables in India. The study provides insights into the HIV-1-specific humoral response in Indian subjects in the context of their antibody titers to two key neutralization targets on the envelope glycoprotein, and this will be helpful for HIV-1 vaccine research.
Material and methods
Study subjects
Ninety-nine HIV-1-seropositive patients enrolled in this study were recruited from the Regional STD Teaching Training and Research Centre, Safdarjang Hospital, New Delhi, India. Whole blood from HIV-1-seronegative healthy donors was collected in EDTA vacutainers after obtaining written informed consent from the blood bank, C.N. Centre, AIIMS, New Delhi. The study was approved by the institute ethics committee, and informed consent was obtained from all the participants.
Peptides
A 35-mer consensus-C V3 (CTRPNNNTRKSIRIGPGQTFYATGDIIGDIRQAHC) and 25-mer consensus-C MPER (DLLALDSWKNLWNWFDITNWLWYIK) peptides were synthesized by Sigma Genosys, USA. The peptides were HPLC-purified to >95% purity (based on information provided by company).
Determination of plasma viral load and CD4 count
The plasma viral load was determined commercially (Lifeline Laboratories Green Park, New Delhi, India) using a real-time-PCR-based method. The viral load was represented as RNA copies/ml of plasma. The CD4 counts were determined by flow cytometry.
Quantitation of plasma IgG levels
The total IgG content in all plasma samples was determined using a commercial ELISA kit from RayBiotech, Inc., USA.
Detection of anti-V3 and anti-MPER antibodies by enzyme-linked immunosorbent assay (ELISA)
The V3 and MPER peptides were immobilized on 96-well high-binding ELISA plates (Corning) by an overnight incubation at 4°C of 100 μl of peptide per well, diluted at 1 μg/ml for V3 and an equimolar concentration for MPER in 50 mM bicarbonate buffer (pH 9.6). Unbound peptides were removed by washing three times with phosphate-buffered saline (PBS) containing 0.2% Tween 20 (Sigma-Aldrich) (wash buffer), and the plates were blocked with 200 μl of Roswell Park Memorial Institute (RPMI) medium containing 15% foetal calf serum (FCS) (Hyclone) and 2% bovine serum albumin (BSA) (Sigma-Aldrich) and incubated for 1 h at 37°C. Next, the plates were washed again three times, and 100 μl of each plasma at six different dilutions was added (dilution range: 1/300-1/100,000), followed by incubation for 1.5 h at 37°C. After three washings with PBST, the plates were further incubated for 1.5 h at 37°C with 100 μl of alkaline-phosphatase-conjugated anti-human IgG Fc (Southern Biotech) diluted 1/2000 in phosphate-buffered saline and Tween-20 (0.05%) containing 2% BSA. Finally, the bound V3- and MPER-specific antibodies were detected by addition of alkaline phosphatase substrate (Sigma-Aldrich) in 10% diethanolamine buffer, and the colorimetric reaction was stopped by the addition of 6 N NaOH. The optical density was read at 405 nm. The cutoff for 50% binding of plasma antibodies, or IC50 value, was defined as three times the mean OD405 of the twenty healthy seronegative plasma plus three standard deviations.
Statistical analysis
Statistical analysis was performed using Graph Pad Prism 5 for Windows, Graph Pad Software, San Diego, California, USA. A non-linear regression curve straight line was plotted using the method of least squares to determine the IC50. Median reciprocal IC50 binding titers were compared using the Wilcoxon matched pairs test or the Mann-Whitney U test. The Spearman rank test was used to determine the correlation between two variables. A p-value less than 0.05 was considered significant for this study.
Results
Characteristics of HIV-1-infected subjects
The details of the 99 HIV-1-infected patients recruited for this study are summarized in Table 1 (supplementary data). The patients had been infected for different time periods, ranging from a few days to seven years (based on time since first diagnosis). There were 32 females and 67 males within the age range of 20-57 years. Out of 99 patients, 65 were naive and 34 had been on antiretroviral therapy for a period of up to two years. The viral load for the naive patients (n = 53) ranged from 156-2,180,000 RNA copies/ml plasma, while for patients undergoing treatment (n = 18), it was low (range = 49.6-5750 RNA copies/ml), showing the effectiveness of ART in suppressing plasma viremia. A few patients in each group (naive and treated) had a viral load below the detection limit (<47 copies/ml). One of the patients on ART (AIIMS 261), possibly a treatment failure case, showed a high viral load (703,000 RNA copies/ml). The mean CD4 count was 288 and 289 cells/cubic millimetre, while the median total IgG levels were 12.31 and 12.05 mg/ml for naive and treated patients, respectively (Supplementary Fig. 2).
Relative reactivity of polyclonal plasma antibodies from HIV-1-infected individuals with consensus clade-C V3 and MPER peptides
The relative antibody titers against the V3 loop and MPER were determined by ELISA binding assays in which plasma antibodies were titrated in a dilution range of 300 to 100,000. The 50% binding titers for both V3 and MPER are shown in Fig. 1, which reveals a high immunogenicity of V3 compared to MPER (p < 0.0001) (Fig. 2). High reactivity of anti-V3 antibodies 96.96% (96/99) was observed in essentially all of the plasma samples with a wide range of IC50 binding titers (446-62,225). Only 3.03% of the plasma (3/99) failed to yield an IC50 value for V3. In comparison, 65.65% (65/99) of the polyclonal plasma samples reacted with the MPER peptide with comparatively lower antibody binding titers (IC50 range: 301-11,543), while 34.34% (34/99) did not yield an IC50 value. The mean IC50 values of all of the plasma samples for V3 and MPER were 10,675 and 1122 respectively.
Relative anti-V3 and anti-MPER antibody titers in HIV-1-infected plasma. 50% binding of polyclonal anti-V3 and anti-MPER antibodies to consensus clade-C V3 loop (above) and MPER (below) peptides. y-axis, IC50 values; x-axis, sample IDs. The total number of samples was 99 (202-300). The IC50 values for the V3 loop and MPER antibodies are shown in vertical bars. The striped bars are samples from patients on ART
Association of V3- and MPER-specific antibody titers with drug status and CD4 counts of patients
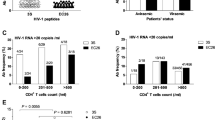
To evaluate the effect of clinical parameters on the antibody-mediated humoral response, we compared anti-V3 and anti-MPER IC50 antibody titers with drug status, CD4 count and total IgG levels. The total IgG levels were comparable between the drug-naive and treated patients (Supplementary Fig. 2). Based on drug status (naive, treated) and CD4 count (CD4 > 300 and <300), the patient samples were grouped (Fig. 3), and their anti-V3 and anti-MPER binding titers were determined separately. We observed significantly lower (p < 0.0001) anti-V3 antibody titers in drug-treated patients than in drug-naive patients, while anti-MPER antibody titers were comparable (p = 0.3413) (Fig. 3). Furthermore, we did not observe any association between CD4 counts and immunogenicity of either regions (V3 and MPER; p = 0.646 and p = 0.394 for naive and treated respectively) (Fig. 3).
Statistical analysis. Comparison of Immunodominance of V3 versus MPER and its association with clinical parameters (drug status and CD4 count). Anti-V3 and anti-MPER IC50 median antibody titers were compared based on drug status (naive [n = 65] vs treated [n = 34], left) and CD4 counts (<300 [n = 58] vs >300 [n = 41]; right) using the Mann-Whitney test. The data were further compared using the Wilcoxon matched pairs test for immunodominance of V3 versus MPER in separate sets (naive V3 vs MPER [n = 65], treated V3 vs MPER [n = 34], CD4 < 300 V3 vs MPER [n = 58], CD4 > 300 V3 vs MPER [n = 41]). The p values for each category are shown in the figure (numerical)
In addition, we did a separate statistical comparison of the immunogenicity of V3 versus MPER using a paired t-test (naive, n = 65, p < 0.0001; treated, n = 34, p < 0.0001; CD4 < 300, n = 58, p < 0.0001; and CD4 > 300, n = 41, p < 0.0001). We observed a complete immunodominance of V3 over MPER, irrespective of drug status and CD4 count (Fig. 3).
Correlation of the plasma antibodies directed to V3 and MPER with plasma viral load
The observation of lower anti-V3 antibody titers in drug-treated as compared to drug-naive patients suggests the influence of virus on antibody titers, since the immediate effect of ART is to reduce the plasma viral load. We evaluated such a possibility by correlating the IC50 titers of both V3 and MPER with plasma viral load (Fig. 4). In order to determine the influence of viremia (independent of drug) on antibody titers, we compared only naive patients (n = 53). Statistical analysis revealed a modest positive correlation (r = 0.2862, p = 0.0377) between titers of V3 antibodies and plasma viral load, suggesting the dependence of anti-V3 antibodies on viral persistence, while MPER-directed antibodies showed a slight negative correlation (r = −0.1247, p = 0.3737).
Effect of ART on V3 and MPER-specific antibody titers (follow-up patients)
We observed an effect of ART on anti-V3 antibody titers, but not anti-MPER antibodies; however, the set of HIV-1-infected individuals (naive or treated) for comparison was different. To confirm our results, we followed up four (AIIMS 118, 149, 151 and 154) HIV-1-infected individuals before and after initiation of ART at three to four time points (2-3 month intervals) for antibodies binding to V3 and MPER.
The 50% binding titers (Fig. 5) show a gradual decrease of anti-V3 antibodies with commencement of ART with respect to time, except in AIIMS 118, who showed an invariable response and a decrease followed by a slight increase at first and second follow-up respectively. The 50% anti-MPER antibody binding titers could not be measured for AIIMS 149 and 154; AIIMS 118 showed a slight decrease, while AIIMS 151 remained relatively constant at all time points.
Effect of ART on V3 and MPER antibody titers (follow-up [FU]). Four HIV-1-positive samples (AIIMS-118, 149, 151 and 154) are presented in the figure, with 2-3-month ART depicted in bars (naive, black filled; first FU, unfilled; second FU, gray filled; third FU, gray striped). A Binding to V3. B Binding to MPER
Association of V3- and MPER-specific antibodies with duration of ART and time from first diagnosis
To assess if there is any correlation between 50% binding titers of anti-V3 and anti-MPER antibodies and duration of ART, we compared V3- and MPER-specific antibody titers of 33 treated patients with duration of their drug therapy (Supplementary Fig. 1). In addition, we compared the same with days from first diagnosis in 64 drug-naive patients (Supplementary Fig. 1). Neither of these factors (ART duration, days from first diagnosis) showed any correlation with antibody titers against either region (V3 or MPER).
Discussion
Relevant information about antibody correlates specific to important regions of HIV-1 will be crucial for a prophylactic vaccine. The present study broadly focuses on the relative antibody titers directed to V3 and MPER, the two important targets of neutralization on the HIV-1 envelope glycoprotein. Plasma samples from 99 HIV-1-infected individuals from North India were tested for the presence of anti-V3 and anti-MPER polyclonal antibodies. This is the first study to provide an indirect estimate of the frequency of V3- and MPER-specific antibody producing B-cells in Indian HIV-1-infected individuals. Based on the polyclonal antibody reactivity of HIV-1-infected plasma to V3 and MPER consensus-C peptides, we observed immunodominance of the V3 loop of gp120 over MPER of gp41. Among the clinical and immunological parameters tested (drug status, CD4 count, total IgG levels and viral load) for association with V3- and MPER-specific antibody titers, we observed a positive correlation of polyclonal anti-V3 Abs with plasma viremia.
The 50% binding titers of V3-specific polyclonal plasma antibodies showed that the majority (96.96%) of the HIV-1-infected plasma samples bound strongly (median IC50 = 6712) to the 35-mer consensus-C V3 peptide, consistent with previous findings [35]. Only three plasma samples, AIIMS 256, AIIMS 268 and AIIMS 274, did not yield an IC50 titer. Interestingly, the first two of these patients (AIIMS 256 and AIIMS 268) were on ART, and the third (AIIMS 274), who presumably had been infected recently (based on days from first diagnosis; Supplementary Table 1), had a low plasma viral load (Supplementary Table 1), which possibly might have accounted for the low anti-V3 antibody titers. Contrary to the result with V3, these plasma exhibited relatively low binding (median IC50 = 552), with varying 50% binding titers to the consensus-C MPER peptide, as only 65.65% yielded IC50 titers. Together, the above findings suggest a high immunogenicity of V3 over MPER in Indian HIV-1 (predominantly clade C)-infected patients. Since the prevalence of HIV-1 infection and clinical parameters associated with HIV-1-specific antibodies in plasma have been the subject of much interest recently [36–38], we therefore, examined the association of the relative antibody titers to V3 and MPER with various clinical and immunological parameters. We found significantly lower anti-V3 antibody titers in drug-treated as compared to drug-naive patients (p < 0.0001), while anti-MPER antibodies were comparable (p = 0.3413), which indirectly suggests a dependence of anti-V3 antibody titers, and not of anti-MPER antibodies, on plasma viremia. However, it was expected that the levels of both the anti-V3 and anti-MPER antibodies should decrease with decreasing plasma viremia due to a reduction in B-cell activation by the antigen. In fact, a few studies have shown that anti-gp120 antibodies decrease upon treatment, and a similar trend has also been observed for anti-gp41 antibodies [39]. However, in this study, we did not find any such association of ART with anti-MPER antibodies, while V3 antibodies showed dependence on antigen persistence, suggesting a relatively high exposure of the V3 loop compared to the MPER. Furthermore, the association of anti-MPER antibodies with viral load shows an inverse trend (Fig. 4) to that of V3, suggesting a differential development of antibody responses to these two regions. Also, the existence of high titers of anti-V3 antibodies against decoy epitopes may possibly make this dependence more prominent [40]. We did not find any association of anti-V3 and anti-MPER antibody titers with patient CD4 counts. In addition, the total IgG levels in naive and treated patients were comparable (Supplementary Fig. 2), suggesting a viral-antigen-specific action of ART, unlike what was previously thought about the sensitivity of non-specific B cell responses to combinational antiretroviral therapy [41]. These results also suggest that ART is effective without affecting the normal B-cell-mediated humoral immune response.
The association of anti-V3 and anti-MPER antibody titers with the plasma viral load observed in the drug-naive patients is indicative of a positive correlation between anti-V3 antibodies and plasma viremia. Furthermore, measurement of antibody titers in four drug-naive patients and, upon treatment, in their follow-up (3-month intervals) revealed a marked reduction in anti-V3 antibody titers upon drug treatment with respect to time, which implies a strong association of anti-V3 antibody levels and viral load. Furthermore, there was no correlation of anti-V3 antibody titers with the duration of ART, which rules out the time dependence factor. This suggests that the decrease in anti-V3 antibody titers probably occurs as a consequence of reduction in plasma viremia upon treatment. However, this hypothesis could not be confirmed, due to the unavailability of viral load details for the follow-up samples. Unlike V3, the anti-MPER antibodies did not show a positive relationship with plasma viral load, but this inference may not be conclusive because of the low immunogenicity of MPER. In fact, a large sample size in the follow-up category could have addressed that issue, which remains a limitation of this study. Our observations suggest the dependence of anti-V3 antibodies on persistence of virus in circulation, whereas no such association was observed for the MPER region. Although statistically not significant, we observed that naive patients with recent infections generally had high levels of anti-V3 antibodies. These high antibody titers persisted throughout the infection in drug-naive patients, suggesting a high affinity maturation of V3 antibodies with persistent virus in the immune system.
Conclusions
Our findings of a high titre of anti-V3 antibodies in Indian HIV-1-infected patients are suggestive of the presence of a substantial number of V3-specific antibody-producing B-cell clones in these patients, in contrast to MPER, possibly due to the low immunogenicity of MPER and selective deletion of B cells with MPER specificity [42, 43]. This work indirectly extends the information about the extent of exposure of V3 and MPER on the native trimeric spike of the virus in HIV-1 subtype-C-infected patients by demonstrating the differential antibody response elicited in these patients to V3 and MPER. Furthermore, it will be interesting to study the association of these parameters with virus neutralization, which can be important for prophylactic vaccine design.
References
Pantophlet R, Burton DR (2006) GP120: target for neutralizing HIV-1 antibodies. Annu Rev Immunol 24:739–769
Zolla-Pazner S (2004) Identifying epitopes of HIV-1 that induce protective antibodies. Nat Rev Immunol 4:199–210
Stamatos NM, Mascola JR, Kalyanaraman VS et al (1998) Neutralizing antibodies from the sera of human immunodeficiency virus type 1-infected individuals bind to monomeric gp120 and oligomeric gp140. J Virol 72:9656–9667
Binley JM, Wrin T, Korber B et al (2004) Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol 78:13232–13252
Li Y, Migueles SA, Welcher B et al (2007) Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat Med 13:1032–1034
Burton DR, Pyati J, Koduri R et al (1994) Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024–1027
Binley JM, Lybarger EA, Crooks ET et al (2008) Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J Virol 82:11651–11668
Stamatatos L, Morris L, Burton DR et al (2009) Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med 15:866–870
Nickle DC, Rolland M, Jensen MA et al (2007) Coping with viral diversity in HIV vaccine design. PLoS Comput Biol 3:e75
Korber B, Gnanakaran S (2009) The implications of patterns in HIV diversity for neutralizing antibody induction and susceptibility. Curr Opin HIV AIDS 4:408–417
Lynch RM, Shen T, Gnanakaran S et al (2009) Appreciating HIV type 1 diversity: subtype differences in Env. AIDS Res Hum Retroviruses 25:237–248
Taylor BS, Hammer SM (2008) The challenge of HIV-1 subtype diversity. N Engl J Med 359:1965–1966
Letvin NL (2002) Strategies for an HIV vaccine. J Clin Invest 110:15–20
McMichael AJ (2006) HIV vaccines. Annu Rev Immunol 24:227–255
Hemelaar J, Gouws E, Ghys PD et al (2006) Global and regional distribution of HIV-1 genetic subtypes and recombinants in 2004. AIDS 20:W13–W23
Berger EA, Murphy PM, Farber JM (1999) Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol 17:657–700
Thali M, Moore JP, Furman C et al (1993) Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol 67:3978–3988
Dalgleish AG, Beverley PC, Clapham PR et al (1984) The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763–767
Deng H, Liu R, Ellmeier W et al (1996) Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661–666
Dragic T, Litwin V, Allaway GP et al (1996) HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667–673
Wyatt R, Sodroski J (1998) The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884–1888
Lu M, Blacklow SC, Kim PS (1995) A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat Struct Biol 2:1075–1082
Mascola JR, Montefiori DC (2010) The role of antibodies in HIV vaccines. Annu Rev Immunol 28:413–444
Zolla-Pazner S, Cohen SS, Krachmarov C et al (2008) Focusing the immune response on the V3 loop, a neutralizing epitope of the HIV-1 gp120 envelope. Virology 372:233–246
Jiang X, Burke V, Totrov M et al (2010) Conserved structural elements in the V3 crown of HIV-1 gp120. Nat Struct Mol Biol 17:955–961
Almond D, Kimura T, Kong X et al (2010) Structural conservation predominates over sequence variability in the crown of HIV type 1’s V3 loop. AIDS Res Hum Retroviruses 26:717–723
Zhu P, Liu J, Bess J Jr et al (2006) Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 441:847–852
Buchacher A, Predl R, Strutzenberger K et al (1994) Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein–Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses 10:359–369
Muster T, Steindl F, Purtscher M et al (1993) A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol 67:6642–6647
Zwick MB, Labrijn AF, Wang M et al (2001) Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol 75:10892–10905
Krachmarov C, Pinter A, Honnen WJ et al (2005) Antibodies that are cross-reactive for human immunodeficiency virus type 1 clade A and clade B v3 domains are common in patient sera from Cameroon, but their neutralization activity is usually restricted by epitope masking. J Virol 79:780–790
Smith JD, Bruce CB, Featherstone AS et al (1994) Reactions of Ugandan antisera with peptides encoded by V3 loop epitopes of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses 10:577–583
Geffin RB, Scott GB, Melenwick M et al (1998) Association of antibody reactivity to ELDKWA, a glycoprotein 41 neutralization epitope, with disease progression in children perinatally infected with HIV type 1. AIDS Res Hum Retroviruses 14:579–590
Sahni AK, Prasad VV, Seth P (2002) Genomic diversity of human immunodeficiency virus type-1 in India. Int J STD AIDS 13:115–118
Gorny MK, Revesz K, Williams C et al (2004) The v3 loop is accessible on the surface of most human immunodeficiency virus type 1 primary isolates and serves as a neutralization epitope. J Virol 78:2394–2404
Nokta M, Turk P, Loesch K et al (2000) Neutralization profiles of sera from human immunodeficiency virus (HIV)-infected individuals: relationship to HIV viral load and CD4 cell count. Clin Diagn Lab Immunol 7:412–416
Piantadosi A, Panteleeff D, Blish CA et al (2009) Breadth of neutralizing antibody response to human immunodeficiency virus type 1 is affected by factors early in infection but does not influence disease progression. J Virol 83:10269–10274
Sather DN, Armann J, Ching LK et al (2009) Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol 83:757–769
Muhlbacher M, Spruth M, Siegel F, Zangerle R, Dierich MP (1999) Longitudinal study of antibody reactivity against HIV-1 envelope and a peptide representing a conserved site on Gp41 in HIV-1-infected patients. Immunobiology 200:295–305
Garrity RR, Rimmelzwaan G, Minassian A, Tsai WP, Lin G, de Jong JJ, Goudsmit J, Nara PL (1997) Refocusing neutralizing antibody response by targeted dampening of an immunodominant epitope. J Immunol 159:279–289
Morris L, Binley JM, Clas BA, Bonhoeffer S, Astill TP, Kost R, Hurley A, Cao Y, Markowitz M, Ho DD, Moore JP (1998) HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J Exp Med 188:233–245
Haynes BF, Fleming J, St Clair EW et al (2005) Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 308:1906–1908
Nabel GJ (2005) Immunology. Close to the edge: neutralizing the HIV-1 envelope. Science 308:1878–1879
Acknowledgments
We profoundly thank all the study participants. We acknowledge Prof. Miroslaw K. Gorny and Prof. Susan Zolla Pazner for their constant technical advice and support. We thank DBT (BT/PR 10511/MED/29/66/2008) and ICMR (61/7/2008-BMS) for funding this work. The AITRP fellowship (USA), JRF fellowship provided by ICMR is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Andrabi, R., Choudhary, A.K., Bala, M. et al. Relative reactivity of HIV-1 polyclonal plasma antibodies directed to V3 and MPER regions suggests immunodominance of V3 over MPER and dependence of high anti-V3 antibody titers on virus persistence. Arch Virol 156, 1787–1794 (2011). https://doi.org/10.1007/s00705-011-1053-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-011-1053-5