Abstract
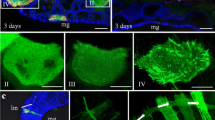
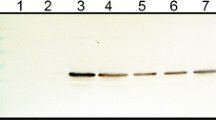
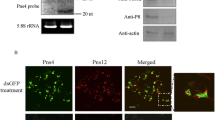
Southern rice black-streaked dwarf virus (SRBSDV), an insect- and plant-infecting reovirus of the genus Fijivirus, induced the formation of virus-containing tubules in infected plant and insect vector cells. Expression of the nonstructural protein P7-1 of SRBSDV in insect cells by a recombinant baculovirus resulted in the formation of tubules with dimensions and appearance similar to those found in SRBSDV-infected cells. These tubules protruded from the cell surface, supporting the hypothesis that the P7-1 protein contains two putative transmembrane domains that are necessary for the formation of tubules. Furthermore, the self-interaction of SRBSDV P7-1 protein indicates that this protein has the capacity to form homodimers or oligomers to assemble the proposed helical symmetry structure of tubules. Taken together, our results indicate that SRBSDV P7-1 has the intrinsic ability of self-interaction to form tubules growing from the cell surface in the absence of other viral proteins.




Similar content being viewed by others
References
Milne RG, del Vas M, Harding RM, Marzachì R, Mertens PPC. Fijivirus, pp 534–542; Omura T, Mertens PPC. Phytoreovirus, pp 543–549; Upadhyaya NM, Mertens PPC. Oryzavirus, pp 550–555 (all 2005). In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (eds) Virus taxonomy: Classification and nomenclature of viruses, Eighth report of the international committee on taxonomy of viruses. Elsevier, Academic Press; Amsterdam, Holland, pp 450–606
Shikata E (1969) Electron microscopic studies on rice viruses: the virus disease of the rice plant. Johns Hopkins University Press, Baltimore, pp 223–240
Hibino H (1996) Biology and epidemiology of rice viruses. Annu Rev Phytopathol 34:249–274
Isogai M, Uyeda I, Lee BC (1998) Detection and assignment of proteins encoded by rice black streaked dwarf fijivirus S7, S8, S9 and S10. J Gen Virol 79:1487–1494
Zhou G, Wen J, Cai D, Li P, Xu D, Zhang S (2008) Southern rice black-streaked dwarf virus: a new proposed Fijivirus species in the family Reoviridae. Chin Sci Bull 53:3677–3685
Zhang HM, Yang J, Chen JP, Adams MJ (2008) A black-streaked dwarf disease on rice in China is caused by a novel Fijivirus. Arch Virol 153:1893–189
Wang Q, Yang J, Zhou G, Zhang HM, Chen JP, Adams MJ (2010) The complete genome sequence of two isolates of southern rice black-streaked dwarf virus, a new member of the Genus Fijivirus. J Phytopathol 158:733–737
Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song K, Pikaard CS (2006) Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45:616–629
Wei T, Huang TS, McNeil J, Laliberté JF, Hong J, Nelson RS, Wang A (2010) Sequential recruitment of the endoplasmic reticulum and chloroplasts for plant potyvirus replication. J Virol 84:799–809
Wei T, Zhang C, Hong J, Xiong R, Kasschau KD, Zhou X, Carrington JC, Wang A (2010) Formation of complexes at plasmodesmata for potyvirus intercellular movement is mediated by the viral protein P3N-PIPO. Plos Pathog 6:e1000962
Rost B, Casadio R, Fariselli P (1996) Refining neural network predictions for helical transmembrane proteins by dynamic programming. Proc Int Conf Intell Syst Mol Biol 4:192–200
Hofmann K, Stoffel W (1993) TMbase—a database of membrane spanning protein segments. Biol Chem 347:166
Sonnhammer EL, von Heijne G, Krogh A (1998) A hidden markov model for predicting transmembrane helices in protein sequences. Proc Int Conf Intell Syst Mol Biol 6:175–182
Atanassov II, Atanassov II, Etchells JP, Turner SR (2009) A simple, flexible and efficient PCR-fusion/Gateway cloning procedure for gene fusion, site-directed mutagenesis, short sequence insertion and domain deletions and swaps. Plant Methods 5:14
Szewczyk E, Nayak T, Oakley CE, Edgerton H, Xiong Y, Taheri-Talesh N, Osmani SA, Oakley BR (2006) Fusion PCR and gene targeting in Aspergillus nidulans. Nat Protoc 1:3111–3120
Monastyrskaya K, Booth T, Nel L, Roy P (1994) Mutation of either of two cysteine residues or deletion of the amino or carboxy terminus of nonstructural protein NS1 of bluetongue virus abrogates virus-specified tubule formation in insect cells. J Virol 68:2169–2178
Wei T, Kikuchi A, Moriyasu Y, Suzuki N, Shimizu T, Hagiwara K, Chen H, Takahashi M, Ichiki-Uehara T, Omura T (2006) The spread of Rice dwarf virus among cells of its insect vector exploits virus-induced tubular structures. J Virol 80:8593–8602
Sparkes IA, Runions J, Kearns A, Hawes C (2006) Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat Protoc 1:2019–2025
Katayama S, Wei T, Omura T, Takagi J, Iwasaki K (2007) Three-dimensional architecture of virus-packed tubule. J Electron Microsc 56:77–81
Ritzenthaler C, Hofmann C (2007) Tubule-guided movement of plant viruses. Plant Cell Monogr 7:64–83
Wei T, Shimizu T, Omura T (2008) Endomembranes and myosin mediate assembly into tubules of Pns10 of Rice dwarf virus and intercellular spreading of the virus in cultured insect vector cells. Virology 372:349–356
Pu Y, Kikuchi A, Moriyasu Y et al (2011) Rice dwarf viruses with dysfunctional genomes generated in plants are filtered out in vector insects—implications for the virus origin. J Virol 85:2975–2979
Nasu S (1965) Electron microscopy studies on transovarial passage of rice dwarf virus. Jpn J Appl Entomol Zool 9:225–237
Owens RJ, Limn C, Roy P (2004) Role of an arbovirus nonstructural protein in cellular pathogenesis and virus release. J Virol 78:6649–6656
Acknowledgments
This work was supported by grants from the programs for the National Basic Research Program of China (No. 2010CB126203), Specialized Research Fund for the Ministry of Agriculture (No. 201003031), New Century Excellent Talents in University (No. NCET-09-0011), the National Natural Science Foundation of China (No. 31070130) and the Natural Science Foundation of Fujian Province (No. 2010J01075).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Y. Liu and D. Jia contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Schematic diagram of the use of fusion PCR to synthesize the fragments for creating mutant P7-1 in which the TM1 or TM2 segment was deleted. (A) Segment 1-107 and segment 127-358 were amplified with primers P1 and P2, and primers P3 and P4, respectively. P2 and P3 are complementary PCR primers consisting of two fused sections (shown in red and blue) that match regions flanking the deleted domain. The two fragments of PCR products were mixed and fused together by PCR using primers P1 and P4, creating mutant P7-1, in which the TM1 was deleted. (B) Segment 1-285 and segment 304-358 were amplified with primers P1 and P5, and primers P6 and P4, respectively. P5 and P6 are complementary PCR primers consisting of two fused sections (shown in red and blue) that match regions flanking the deleted domain. The two fragments of the PCR products were mixed and fused together by PCR using primers P1 and P4, creating mutant P7-1, in which TM2 was deleted. (TIFF 134 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Jia, D., Chen, H. et al. The P7-1 protein of southern rice black-streaked dwarf virus, a fijivirus, induces the formation of tubular structures in insect cells. Arch Virol 156, 1729–1736 (2011). https://doi.org/10.1007/s00705-011-1041-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-011-1041-9