Abstract
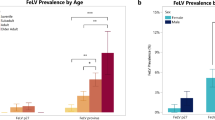
The Iberian lynx is the most endangered felid species. During winter/spring 2006/7, a feline leukemia virus (FeLV) outbreak of unexpected virulence killed about 2/3 of the infected Iberian lynxes. All FeLV-positive animals were co-infected with feline hemoplasmas. To further characterize the Iberian lynx FeLV strain and evaluate its potential virulence, the FeLV envelope gene variable region A (VRA) mutant spectrum was analyzed using the Roche 454 sequencing technology, and an in vivo transmission study of lynx blood to specified-pathogen-free cats was performed. VRA mutations indicated weak apolipoprotein B mRNA editing enzyme and catalytic polypeptide-like cytidine deaminase (APOBEC) restriction of FeLV replication, and variants characteristic of aggressive FeLV strains, such as FeLV-C or FeLV-A/61C, were not detected. Cats exposed to FeLV/Candidatus Mycoplasma haemominutum-positive lynx blood did not show a particularly severe outcome of infection. The results underscore the special susceptibility of Iberian lynxes to infectious diseases.




Similar content being viewed by others
References
Anderson L, Wilson R, Hay D (1971) Haematological values in normal cats from four weeks to one year of age. Res Vet Sci 12:579–583
Anderson MM, Lauring AS, Burns CC, Overbaugh J (2000) Identification of a cellular cofactor required for infection by feline leukemia virus. Science 287:1828–1830
Arnaud F, Caporale M, Varela M, Biek R, Chessa B, Alberti A, Golder M, Mura M, Zhang Y-p, Yu L, Pereira F, DeMartini JC, Leymaster K, Spencer TE, Palmarini M (2007) A paradigm for virus-host coevolution: sequential counter-adaptations between endogenous and exogenous Retroviruses. PLoS Pathog 3:e170
Athas GB, Choi B, Prabhu S, Lobelle-Rich PA, Levy LS (1995) Genetic determinants of feline leukemia virus-induced multicentric lymphomas. Virology 214:431–438
Athas GB, Lobelle-Rich P, Levy LS (1995) Function of a unique sequence motif in the long terminal repeat of feline leukemia virus isolated from an unusual set of naturally occurring tumors. J Virol 69:3324–3332
Brockman MA, Brumme ZL, Brumme CJ, Miura T, Sela J, Rosato PC, Kadie CM, Carlson JM, Markle TJ, Streeck H, Kelleher AD, Markowitz M, Jessen H, Rosenberg E, Altfeld M, Harrigan PR, Heckerman D, Walker BD, Allen TM (2010) Early selection in Gag by protective HLA alleles contributes to reduced HIV-1 replication capacity that may be largely compensated in chronic infection. J Virol 84:11937–11949
Brojatsch J, Kristal BS, Viglianti GA, Khiroya R, Hoover EA, Mullins JI (1992) Feline leukemia virus subgroup C phenotype evolves through distinct alterations near the N terminus of the envelope surface glycoprotein. Proc Natl Acad Sci USA 89:8457–8461
Cattori V, Tandon R, Pepin A, Lutz H, Hofmann-Lehmann R (2006) Rapid detection of feline leukemia virus provirus integration into feline genomic DNA. Mol Cell Probes 20:172–181
Cattori V, Meli ML, Tandon R, Vargas A, Martinez F, Lopez G, Zorilla I, Munoz A, Palomares F, Lopez JV, Hofmann-Lehmann R, Lutz H (2008) Feline Leukemia Virus (FeLV) outbreak in Iberian lynxes: proviral env sequence analysis and endogenous FeLV quantification. In: 9th International Feline Retrovirus Research Symposium, Vienna
Cattori V, Tandon R, Riond B, Pepin AC, Lutz H, Hofmann-Lehmann R (2009) The kinetics of feline leukaemia virus shedding in experimentally infected cats are associated with infection outcome. Vet Microbiol 133:292–296
Chandhasin C, Lobelle-Rich P, Levy LS (2004) Feline leukaemia virus LTR variation and disease association in a geographical and temporal cluster. J Gen Virol 85:2937–2942
Chiu Y-L, Greene WC (2008) The APOBEC3 cytidine deaminases: an innate defensive network opposing exogenous retroviruses and endogenous retroelements. Ann Rev Immunol 26:317–353
Cleaveland S (2009) Viral threats and vaccination: disease management of endangered species. Animal conservation 12:187–189
Cunningham MW, Brown MA, Shindle DB, Terrell SP, Hayes KA, Ferree BC, McBride RT, Blankenship EL, Jansen D, Citino SB, Roelke ME, Kiltie RA, Troyer JL, O’Brien SJ (2008) Epizootiology and management of feline leukemia virus in the Florida puma. J Wildl Dis 44:537–552
Dewannieux M, Collins MK (2008) Spontaneous heteromerization of gammaretroviral envelope proteins: a possible novel mechanism of retrovirus restriction. J Virol 82:9789–9794
Donahue PR, Hoover EA, Beltz GA, Riedel N, Hirsch VM, Overbaugh J, Mullins JI (1988) Strong sequence conservation among horizontally transmissible, minimally pathogenic feline leukemia viruses. J Virol 62:722–731
Dunham SP, Graham E (2008) Retroviral infections of small animals. Vet Clin North Am Small Anim Pract 38:879-901, ix
Fan J, Ma G, Nosaka K, Tanabe J, Satou Y, Koito A, Wain-Hobson S, Vartanian J-P, Matsuoka M (2010) APOBEC3G generates nonsense mutations in human T-cell leukemia virus type 1 proviral genomes in vivo. J Virol 84:7278–7287
Favrot C, Wilhelm S, Grest P, Meli ML, Hofmann-Lehmann R, Kipar A (2005) Two cases of FeLV-associated dermatoses. Vet Dermatol 16:407–412
Ferreras P, Rodríguez A, Palomares F, Delibes M (2010) Iberian lynx: the uncertain future of a critically endangered cat. In: Macdonald DW, Loveridge AJ (eds) Biology and conservation of wild felids. Oxford University Press, Oxford, pp 511–524
Flynn JN, Dunham SP, Watson V, Jarrett O (2002) Longitudinal analysis of feline leukemia virus-specific cytotoxic T lymphocytes: correlation with recovery from infection. J Virol 76:2306–2315
Fromont E, Sager A, Leger F, Bourguemestre F, Jouquelet E, Stahl P, Pontier D, Artois M (2000) Prevalence and pathogenicity of retroviruses in wildcats in France. Vet Rec 146:317–319
George JW, Rideout BA, Griffey SM, Pedersen NC (2002) Effect of preexisting FeLV infection or FeLV and feline immunodeficiency virus coinfection on pathogenicity of the small variant of Haemobartonella felis in cats. Am J Vet Res 63:1172–1178
Gleich SE, Krieger S, Hartmann K (2009) Prevalence of feline immunodeficiency virus and feline leukaemia virus among client-owned cats and risk factors for infection in Germany. J Feline Med Surg 11:985–992
Gomes-Keller MA (2008) Feline Leukemia Virus infection: new aspects of pathogenesis as a consequence of the infection pressure. Vetsuisse Faculty, Zurich
Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R (2008) Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods 5:235–237
Harrus S, Klement E, Aroch I, Stein T, Bark H, Lavy E, Mazaki-Tovi M, Baneth G (2002) Retrospective study of 46 cases of feline haemobartonellosis in Israel and their relationships with FeLV and FIV infections. Vet Rec 151:82–85
Heyman P, Vaheri A, Lundkvist A, Avsic-Zupanc T (2009) Hantavirus infections in Europe: from virus carriers to a major public-health problem. Expert Rev Anti Infect Ther 7:205–217
Hofmann-Lehmann R, Fehr D, Grob M, Elgizoli M, Packer C, Martenson JS, O’Brien SJ, Lutz H (1996) Prevalence of antibodies to feline parvovirus, calicivirus, herpesvirus, coronavirus, and immunodeficiency virus and of feline leukemia virus antigen and the interrelationship of these viral infections in free-ranging lions in east Africa. Clin Diagn Lab Immunol 3:554–562
Hofmann-Lehmann R, Tandon R, Boretti FS, Meli ML, Willi B, Cattori V, Gomes-Keller MA, Ossent P, Golder MC, Flynn JN, Lutz H (2006) Reassessment of feline leukaemia virus (FeLV) vaccines with novel sensitive molecular assays. Vaccine 24:1087–1094
Hofmann-Lehmann R, Cattori V, Tandon R, Boretti FS, Meli ML, Riond B, Lutz H (2008) How molecular methods change our views of FeLV infection and vaccination. Vet Immunol Immunopathol 123:119–123
Hoover EA, Olsen RG, Hardy WD Jr, Schaller JP, Mathes LE (1976) Feline leukemia virus infection: age-related variation in response of cats to experimental infection. J Natl Cancer Inst 57:365–369
Hoover EA, Mullins JI (1991) Feline leukemia virus infection and diseases. J Am Vet Med Assoc 199:1287–1297
IUCN (2006) IUCN red list of threatened species. http://wwwiucnredlistorg/apps/redlist/details/12520/0
Jarrett O, Laird HM, Hay D (1973) Determinants of the host range of feline leukaemia viruses. J Gen Virol 20:169–175
Jarrett O, Ganiere JP (1996) Comparative studies of the efficacy of a recombinant feline leukaemia virus vaccine. Vet Rec 138:7–11
Johnson WE, Godoy JA, Palomares F, Delibes M, Fernandes M, Revilla E, O’Brien SJ (2004) Phylogenetic and phylogeographic analysis of Iberian lynx populations. J Hered 95:19–28
Karber G (1931) 50% end-point calculation. Arch Exp Pathol Pharmak 162:480–483
Khanna M, Kumar P, Choudhary K, Kumar B, Vijayan VK (2008) Emerging influenza virus: a global threat. J Biosci 33:475–482
Kim E-Y, Bhattacharya T, Kunstman K, Swantek P, Koning FA, Malim MH, Wolinsky SM (2010) Human APOBEC3G-mediated editing can promote HIV-1 sequence diversification and accelerate adaptation to selective pressure. J Virol 84:10402–10405
Laberke S, Just F, Pfister K, Hartmann K (2010) Prevalence of feline haemoplasma infection in cats in Southern Bavaria, Germany, and infection risk factor analysis. Berl Munch Tierarztl Wochenschr 123:42–48
Lappin MR (1995) Opportunistic infections associated with retroviral infections in cats. Semin Vet Med Surg (Small Anim) 10:244–250
Leutenegger CM, Hofmann-Lehmann R, Riols C, Liberek M, Worel G, Lups P, Fehr D, Hartmann M, Weilenmann P, Lutz H (1999) Viral infections in free-living populations of the European wildcat. J Wildl Dis 35:678–686
Liddament MT, Brown WL, Schumacher AJ, Harris RS (2004) APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Curr Biol 14:1385–1391
Lingappa JR, Hughes JP, Wang RS, Baeten JM, Celum C, Gray GE, Stevens WS, Donnell D, Campbell MS, Farquhar C, Essex M, Mullins JI, Coombs RW, Rees H, Corey L, Wald A, for the Partners in Prevention HSVHIVTST (2010) Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS ONE 5:e12598
López G, López-Parra M, Fernández L, Martínez-Granados C, Martínez F, Meli ML, Gil-Sánchez JM, Viqueira N, Díaz-Portero MA, Cadenas R, Lutz H, Vargas A, Simón MA (2009) Management measures to control a feline leukemia virus outbreak in the endangered Iberian lynx. Anim Conserv 12:173–182
Lotscher M, Recher M, Lang KS, Navarini A, Hunziker L, Santimaria R, Glatzel M, Schwarz P, Boni J, Zinkernagel RM (2007) Induced prion protein controls immune-activated retroviruses in the mouse spleen. PLoS ONE 2:e1158
Luaces I, Domenech A, Garcia-Montijano M, Collado VM, Sanchez C, Tejerizo JG, Galka M, Fernandez P, Gomez-Lucia E (2008) Detection of Feline leukemia virus in the endangered Iberian lynx (Lynx pardinus). J Vet Diagn Invest 20:381–385
Lutz H, Pedersen N, Higgins J, Hubscher U, Troy FA, Theilen GH (1980) Humoral immune reactivity to feline leukemia virus and associated antigens in cats naturally infected with feline leukemia virus. Cancer Res 40:3642–3651
Lutz H, Pedersen NC, Durbin R, Theilen GH (1983) Monoclonal antibodies to three epitopic regions of feline leukemia virus p27 and their use in enzyme-linked immunosorbent assay of p27. J Immunol Methods 56:209–220
Lutz H, Pedersen NC, Theilen GH (1983) Course of feline leukemia virus infection and its detection by enzyme-linked immunosorbent assay and monoclonal antibodies. Am J Vet Res 44:2054–2059
Lutz H, Arnold P, Hubscher U, Egberink H, Pedersen N, Horzinek MC (1988) Specificity assessment of feline T-lymphotropic lentivirus serology. Zentralbl Veterinarmed B 35:773–778
Maher IE, Tasker S, Polizopoulou Z, Dasopoulou A, Egan K, Helps CR, Papasouliotis K (2010) Polymerase chain reaction survey of feline haemoplasma infections in Greece. J Feline Med Surg 12:601–605
Major A, Cattori V, Boenzli E, Riond B, Ossent P, Meli ML, Hofmann-Lehmann R, Lutz H (2010) Exposure of cats to low doses of FeLV: seroconversion as the sole parameter of infection. Vet Res 41:17
Mathes LE, Pandey R, Chakrabarti R, Hofman FM, Hayes KA, Stromberg P, Roy-Burman P (1994) Pathogenicity of a subgroup C feline leukemia virus (FeLV) is augmented when administered in association with certain FeLV recombinants. Virology 198:185–195
Meli ML, Cattori V, Martínez F, López G, Vargas A, Palomares F, López-Bao JV, Hofmann-Lehmann R, Lutz H (2010) Feline leukemia virus infection: a threat for the survival of the critically endangered Iberian lynx (Lynx pardinus). Vet Immunol Immunopathol 134:61–67
Millan J, Candela MG, Palomares F, Cubero MJ, Rodriguez A, Barral M, de la Fuente J, Almeria S, Leon-Vizcaino L (2009) Disease threats to the endangered Iberian lynx (Lynx pardinus). Vet J 182:114–124
Moroni C, Schumann G (1977) Are endogenous C-type viruses involved in the immune system? Nature 269:600–601
Munk C, Beck T, Zielonka J, Hotz-Wagenblatt A, Chareza S, Battenberg M, Thielebein J, Cichutek K, Bravo I, O’Brien S, Lochelt M, Yuhki N (2008) Functions, structure, and read-through alternative splicing of feline APOBEC3 genes. Genome Biol 9:R48
Museux K, Boretti FS, Willi B, Riond B, Hoelzle K, Hoelzle LE, Wittenbrink MM, Tasker S, Wengi N, Reusch CE, Lutz H, Hofmann-Lehmann R (2009) In vivo transmission studies of ‘Candidatus Mycoplasma turicensis’ in the domestic cat. Vet Res 40:45
Ostfeld RS (2009) Biodiversity loss and the rise of zoonotic pathogens. Clin Microbiol Infect 15:40–43
Ostrowski S, Van Vuuren M, Lenain DM, Durand A (2003) A serologic survey of wild felids from central west Saudi Arabia. J Wildl Dis 39:696–701
Overbaugh J, Hoover EA, Mullins JI, Burns DP, Rudensey L, Quackenbush SL, Stallard V, Donahue PR (1992) Structure and pathogenicity of individual variants within an immunodeficiency disease-inducing isolate of FeLV. Virology 188:558–569
Peña L, Garcia P, Jimenez MA, Benito A, Perez Alenza MD, Sanchez B (2006) Histopathological and immunohistochemical findings in lymphoid tissues of the endangered Iberian lynx (Lynx pardinus). Comp Immunol Microbiol Infect Dis 29:114–126
Perlman S, Netland J (2009) Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol 7:439–450
Peterson PK, Chao CC, Molitor T, Murtaugh M, Strgar F, Sharp BM (1991) Stress and pathogenesis of infectious disease. Rev Infect Dis 13:710–720
Petit V, Guétard D, Renard M, Keriel A, Sitbon M, Wain-Hobson S, Vartanian J-P (2009) Murine APOBEC1 is a powerful mutator of retroviral and cellular RNA in vitro and in vivo. J Mol Biol 385:65–78
Polas PJ, Swenson CL, Sams R, Cheney CM, Hayes KA, Tarr MJ, Kociba GJ, Mathes LE (1990) In vitro and in vivo evidence that the antiviral activity of 2’, 3’-dideoxycytidine is target cell dependent in a feline retrovirus animal model. Antimicrob Agents Chemother 34:1414–1421
Prabhu S, Lobelle-Rich PA, Levy LS (1999) The FeLV-945 LTR confers a replicative advantage dependent on the presence of a tandem triplication. Virology 263:460–470
Ramsauer S, Bay G, Meli M, Hofmann-Lehmann R, Lutz H (2007) Seroprevalence of selected infectious agents in a free-ranging, low-density lion population in the Central Kalahari Game Reserves in Botswana. Clin Vaccine Immunol 14:808–810
Roelke M, Johnson W, Millán J, Palomares F, Revilla E, Rodríguez A, Calzada J, Ferreras P, León-Vizcaíno L, Delibes M, O’Brien S (2008) Exposure to disease agents in the endangered Iberian lynx (Lynx pardinus). Eur J Wildl Res 54:171–178
Rohn JL, Linenberger ML, Hoover EA, Overbaugh J (1994) Evolution of feline leukemia virus variant genomes with insertions, deletions, and defective envelope genes in infected cats with tumors. J Virol 68:2458–2467
Rojko JL, Hoover EA, Quackenbush SL, Olsen RG (1982) Reactivation of latent feline leukaemia virus infection. Nature 298:385–388
Rojko JL, Kociba GJ (1991) Pathogenesis of infection by the feline leukemia virus. J Am Vet Med Assoc 199:1305–1310
Roura X, Peters IR, Altet L, Tabar M-D, Barker EN, Planellas M, Helps CR, Francino O, Shaw SE, Tasker S (2010) Prevalence of hemotropic mycoplasmas in healthy and unhealthy cats and dogs in Spain. J Vet Diagn Invest 22:270–274
Shalev Z, Duffy SP, Adema KW, Prasad R, Hussain N, Willett BJ, Tailor CS (2009) Identification of a feline leukemia virus variant that can use THTR1, FLVCR1, and FLVCR2 for infection. J Virol 83:6706–6716
Sheets RL, Pandey R, Jen WC, Roy-Burman P (1993) Recombinant feline leukemia virus genes detected in naturally occurring feline lymphosarcomas. J Virol 67:3118–3125
Stern MA, Hu C, Saenz DT, Fadel HJ, Sims O, Peretz M, Poeschla EM (2010) Productive Replication of vif-Chimeric HIV-1 in Feline Cells. J Virol 84:7378–7395
Tandon R, Cattori V, Gomes-Keller MA, Meli ML, Golder MC, Lutz H, Hofmann-Lehmann R (2005) Quantitation of feline leukaemia virus viral and proviral loads by TaqMan® real-time polymerase chain reaction. J Virol Methods 130:124–132
Tasker S, Lappin MR (2002) Haemobartonella felis: recent developments in diagnosis and treatment. J Feline Med Surg 4:3–11
Tasker S, Caney SM, Day MJ, Dean RS, Helps CR, Knowles TG, Lait PJ, Pinches MD, Gruffydd-Jones TJ (2006) Effect of chronic feline immunodeficiency infection, and efficacy of marbofloxacin treatment, on ‘Candidatus Mycoplasma haemominutum’ infection. Microbes Infect 8:653–661
Tasker S, Peters IR, Papasouliotis K, Cue SM, Willi B, Hofmann-Lehmann R, Gruffydd-Jones TJ, Knowles TG, Day MJ, Helps CR (2009) Description of outcomes of experimental infection with feline haemoplasmas: Copy numbers, haematology, Coombs’ testing and blood glucose concentrations. Vet Microbiol 139:323–332
Tasker S, Murray JK, Knowles TG, Day MJ (2010) Coombs’ haemoplasma and retrovirus testing in feline anaemia. J Small Animal Pract 51:192–199
Virtue ER, Marsh GA, Wang LF (2009) Paramyxoviruses infecting humans: the old, the new and the unknown. Futur Microbiol 4:537–554
Weissenbacher S, Riond B, Hofmann-Lehmann R, Lutz H (2010) Evaluation of a novel haematology analyser for use with feline blood. Vet J [Epub ahead of print]
Willi B, Boretti FS, Cattori V, Tasker S, Meli ML, Reusch C, Lutz H, Hofmann-Lehmann R (2005) Identification, molecular characterization, and experimental transmission of a new hemoplasma isolate from a cat with hemolytic anemia in Switzerland. J Clin Microbiol 43:2581–2585
Willi B, Boretti FS, Baumgartner C, Tasker S, Wenger B, Cattori V, Meli ML, Reusch CE, Lutz H, Hofmann-Lehmann R (2006) Prevalence, risk factor analysis, and follow-up of infections caused by three feline hemoplasma species in cats in Switzerland. J Clin Microbiol 44:961–969
Willi B, Filoni C, Catao-Dias JL, Cattori V, Meli ML, Vargas A, Martinez F, Roelke ME, Ryser-Degiorgis MP, Leutenegger CM, Lutz H, Hofmann-Lehmann R (2007) Worldwide occurrence of feline hemoplasma infections in wild felid species. J Clin Microbiol 45:1159–1166
Woodman Z, Williamson C (2009) HIV molecular epidemiology: transmission and adaptation to human populations. Curr Opin HIV AIDS 4:247–252
Acknowledgments
The authors are indebted to the Environmental Council of the Government of Andalusia, southern Spain, for providing the Iberian lynx samples. The lynxes used in the present study were trapped, radio-tracked and sampled as part of research projects CGL2004-00346/BOS of the Spanish Ministry of Education and Science and 17/2005 of the Spanish Ministry of the Environment under the National Parks research program, with the collaboration of Land Rover España S.A. We acknowledge support by the Promedica Foundation, Chur, Switzerland. Laboratory work was performed using the logistics of the Center for Clinical Studies at the Vetsuisse Faculty of the University of Zurich. This study was conducted by C.G. as partial fulfillment of the requirement of a doctoral thesis at the Vetsuisse Faculty of the University of Zurich. V.C. is the recipient of a research grant from the University of Zurich (Forschungskredit 2009). R.H.L. is the recipient of a professorship from the Swiss National Science Foundation (PP00P3-119136). The project was supported by the University of Zurich, Switzerland, by financing the operational activities of the clinical laboratory of the Vetsuisse Faculty, Zurich. We also thank the Functional Genomics Center Zurich for generous support with next-generation sequencing resources. We thank Dr. O. Jarrett, who kindly provided the FeLV-A/Glasgow-1 virus stock, and T. Meili, M. Rios, D. Brasser, M. Künzli and R. Bruggmann for excellent assistance. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. P. Geret, V. Cattori contributed equally to this project and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Geret, C.P., Cattori, V., Meli, M.L. et al. Feline leukemia virus outbreak in the critically endangered Iberian lynx (Lynx pardinus): high-throughput sequencing of envelope variable region A and experimental transmission. Arch Virol 156, 839–854 (2011). https://doi.org/10.1007/s00705-011-0925-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-011-0925-z