Abstract
The hepatitis C virus (HCV) envelope glycoproteins have been shown to cause ER stress and induce the unfolded protein response (UPR). Using a bicistronic reporter, we show that the envelope glycoproteins repressed both cap-dependent and HCV IRES-mediated translation in HeLa cells but displayed a differential repression of cap-dependent translation in Huh-7 cells. In contrast, the envelope glycoproteins repressed E2F transcriptional activity in both HeLa and Huh-7 cells and caused increased accumulation of the underphosphorylated retinoblastoma protein. Expression of the envelope glycoproteins induced eIF2α phosphorylation, suggesting a role of the UPR in regulating translation and E2F transcriptional activity. The envelope glycoproteins also enhanced transcriptional activity from the COX-2 promoter and endogenous COX-2 expression in HeLa cells, but not in Huh-7 cells. Together, these results suggest that the envelope glycoproteins may assume more functional roles in viral replication and host cell interactions.







Similar content being viewed by others
References
Bennett MR, Macdonald K, Chan SW, Boyle JJ, Weissberg PL (1998) Cooperative interactions between RB and p53 regulate cell proliferation, cell senescence, and apoptosis in human vascular smooth muscle cells from atherosclerotic plaques. Circulation Res 82:704–712
Calfon M, Zeng HQ, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92–96
Chan SW, Egan PA (2005) Hepatitis C virus envelope proteins regulate CHOP via induction of the unfolded protein response. FASEB J 19:1510–1512
Collier AJ, Tang S, Elliott RM (1998) Translation efficiencies of the 5′ untranslated region from representatives of the six major genotypes of hepatitis C virus using a novel bicistronic reporter assay system. J Gen Virol 79:2359–2366
Dubuisson J, Hsu HH, Cheung RC, Greenberg HB, Russell DG, Rice CM (1994) Formation and intracellular localization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia and Sindbis viruses. J Virol 68:6147–6160
Dubuisson J, Rice CM (1996) Hepatitis C virus glycoprotein folding: disulfide bond formation and association with calnexin. J Virol 70:778–786
Hamanaka RB, Bennett BS, Cullinan SB, Diehl JA (2005) PERK and GCN2 contribute to eIF2 alpha phosphorylation and cell cycle arrest after activation of the unfolded protein response pathway. Mol Biol Cell 16:5493–5501
Harada S, Suzuki R, Ando A, Watanabe Y, Yagi S, Miyamura T, Saito I (1995) Establishment of a cell-line constitutively expressing E2 glycoprotein of hepatitis-C virus and humoral response of hepatitis-C patients to the expressed protein. J Gen Virol 76:1223–1231
Harding HP, Zhang YH, Ron D (1999) Protein translation and folding are coupled by an endoplasmic- reticulum-resident kinase. Nature 397:271–274
Hassan M, Ghozan H, Abdel-Kader O (2004) Activation of RB/E2F signaling pathway is required for the modulation of hepatitis C virus core protein-induced cell growth in liver and non-liver cells. Cell Signal 16:1375–1385
Hung JH, Su IJ, Lei HY, Wang HC, Lin WC, Chang WT, Huang WY, Chang WC, Chang YS, Chen CC, Lai MD (2004) Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-kappa B and pp38 mitogen-activated protein kinase. J Biol Chem 279:46384–46392
Jhaveri R, Kundu P, Shapiro AM, Venkatesan A, Dasgupta A (2005) Effect of hepatitis C virus core protein on cellular gene expression: Specific inhibition of cyclooxygenase 2. J Infect Dis 191:1498–1506
Joo MS, Hahn YS, Kwon MJ, Sadikot RT, Blackwell TS, Christman JW (2005) Hepatitis C virus core protein suppresses NF-kappa B activation and cyclooxygenase-2 expression by direct interaction with I kappa B kinase beta. J Virol 79:7648–7657
Liberman E, Fong YL, Selby MJ, Choo QL, Cousens L, Houghton M, Yen TSB (1999) Activation of the grp78 and grp94 promoters by hepatitis C virus E2 envelope protein. J Virol 73:3718–3722
Lu LL, Wei L, Peng GQ, Mu YX, Wu KL, Kang L, Yan XH, Zhu Y, Wu HG (2008) NS3 protein of hepatitis C virus regulates cyclooxygenase-2 expression through multiple signaling pathways. Virol 371:61–70
MacCallum PR, Jack SC, Egan PA, McDermott BT, Elliott RM, Chan SW (2006) Cap-dependent and hepatitis C virus internal ribosome entry site-mediated translation are modulated by phosphorylation of eIF2 alpha under oxidative stress. J Gen Virol 87:3251–3262
Marusawa H, Hijikata M, Chiba T, Shimotohno K (1999) Hepatitis C virus core protein inhibits Fas- and tumor necrosis factor alpha-mediated apoptosis via NF-kappa B activation. J Virol 73:4713–4720
Matsuura Y, Harada S, Suzuki R, Watanabe Y, Inoue Y, Saito I, Miyamura T (1992) Expression of processed envelope protein of hepatitis-C virus in mammalian and insect cells. J Virol 66:1425–1431
Meade EA, McIntyre TM, Zimmerman GA, Prescott SM (1999) Peroxisome proliferators enhance cyclooxygenase-2 expression in epithelial cells. J Biol Chem 274:8328–8334
Moradpour D, Brass V, Gosert R, Wolk B, Blum HE (2002) Hepatitis C: molecular virology and antiviral targets. Trends Mol Med 8:476–482
Munakata T, Nakamura M, Liang YQ, Li K, Lemon SM (2005) Down-regulation of the retinoblastoma tumor suppressor by the hepatitis C virus NS5B RNA-dependent RNA polymerase. Proc Natl Acad Sci USA 102:18159–18164
Nunez O, Fernandez-Martinez A, Majano PL, Apolinario A, Gomez-Gonzalo M, Benedicto I, Lopez-Cabrera M, Bosca L, Clemente G, Garcia-Monzon C, Martin-Sanz P (2004) Increased intrahepatic cyclooxygenase 2, matrix metalloproteinase 2, and matrix metalloproteinase 9 expression is associated with progressive liver disease in chronic hepatitis C virus infection: role of viral core and NS5A proteins. Gut 53:1665–1672
Patrignani P, Tacconelli S, Sciulli MG, Capone ML (2005) New insights into COX-2 biology and inhibition. Brain Res Rev 48:352–359
Pavio N, Taylor DR, Lai MM (2002) Detection of a novel unglycosylated form of hepatitis C virus E2 envelope protein that is located in the cytosol and interacts with PKR. J Virol 76:1265–1272
Pavio N, Romano PR, Graczyk TM, Feinstone SM, Taylor DR (2003) Protein synthesis and endoplasmic reticulum stress can be modulated by the hepatitis C virus envelope protein E2 through the eukaryotic initiation factor 2alpha kinase PERK. J Virol 77:3578–3585
Pietschmann T, Lohmann V, Rutter G, Kurpanek K, Bartenschlager R (2001) Characterization of cell lines carrying self-replicating hepatitis C virus RNAs. J Virol 75:1252–1264
Polager S, Ginsberg D (2008) E2F-at the crossroads of life and death. Trends Cell Biol 18:528–535
Pontsler AV, St Hilaire A, Marathe GK, Zimmerman GA, McIntyre TM (2002) Cyclooxygenase-2 is induced in monocytes by peroxisome proliferator activated receptor gamma and oxidized alkyl phospholipids from oxidized low density lipoprotein. J Biol Chem 277:13029–13036
Poznic M (2009) Retinoblastoma protein: a central processing unit. J Biosci 34:305–312
Rahman MA, Dhar DK, Yamaguchi E, Maruyama S, Sato T, Hayashi H, Ono T, Yamanoi A, Kohno H, Nagasue N (2001) Coexpression of inducible nitric oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver: possible involvement of COX-2 in the angiogenesis of hepatitis C virus-positive cases. Clin Cancer Res 7:1325–1332
Robert F, Kapp LD, Khan SN, Acker MG, Kolitz S, Kazemi S, Kaufman RJ, Merrick WC, Koromilas AE, Lorsch JR, Pelletier J (2006) Initiation of protein synthesis by hepatitis C virus is refractory to reduced eIF2.GTP.Met-tRNA(i)(Met) ternary complex availability. Mol Biol Cell 17:4632–4644
Sekine-Osajima Y, Sakamoto N, Mishima K, Nakagawa M, Itsui Y, Tasaka M, Nishimura-Sakura Y, Chen CH, Kanai Y, Tsuchiya K, Wakita T, Enomoto N, Watanabe M (2008) Development of plaque assays for hepatitis C virus-JFH1 strain and isolation of mutants with enhanced cytopathogenicity and replication capacity. Virol 371:71–85
Selby M, Erickson A, Dong C, Cooper S, Parham P, Houghton M, Walker CM (1999) Hepatitis C virus envelope glycoprotein E1 originates in the endoplasmic reticulum and requires cytoplasmic processing for presentation by class I MHC molecules. J Immunol 162:669–676
Sir D, Chen WL, Choi J, Wakita T, Yen TS, Ou JH (2008) Induction of incomplete autophagic response by hepatitis C virus via the unfolded protein response. Hepatol 48:1054–1061
Taylor DR, Shi ST, Romano PR, Barber GN, Lai MM (1999) Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science 285:107–110
Terenin IM, Dmitriev SE, Andreev DE, Shatsky IN (2008) Eukaryotic translation initiation machinery can operate in a bacterial-like mode without eIF2. Nat Struct Mol Biol 15:836–841
Wek RC, Jiang HY, Anthony TG (2006) Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34:7–11
Acknowledgments
We thank Charlie Rice for the Huh-7 cell line, Jean Dubuisson for the anti-E1 and anti-E2 antibodies, Makoto Hijikata, Tatsuo Miyamura, Yoshiharu Matsuura, Richard Elliott, Martin Bennett and Thomas McIntyre for plasmids. This work was supported in part by a Medical Research Council Career Establishment grant G0000092 awarded to SWC. Microscopy was performed using a Zeiss epifluorescent microscope and a confocal microscope from a Biotechnology and Biological Sciences Research Council JREI equipment grant (JR00UMJAEQ, JR00UMJARC) to SWC.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
705_2009_495_MOESM1_ESM.ppt
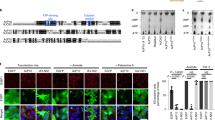
Subcellular localization of E1 and E2 proteins. Fluorescent microscopy showing the subcellular localization of the ER-targeting E1 and E2 and the gfp-E1 and gfp-E2 devoid of signal peptides. Supplementary figure (PPT 1666 kb)
Rights and permissions
About this article
Cite this article
Chan, SW., Egan, P.A. Effects of hepatitis C virus envelope glycoprotein unfolded protein response activation on translation and transcription. Arch Virol 154, 1631–1640 (2009). https://doi.org/10.1007/s00705-009-0495-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-009-0495-5