Abstract
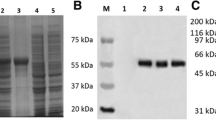
Compared to the UL51 gene of other alphaherpesviruses, the duck enteritis virus (DEV) UL51 gene contains ten conserved motifs and has a close evolutionary relationship with members of the genus Mardivirus. The DEV UL51 gene product was identified using a rabbit polyclonal antiserum raised against a 6-His-UL51 fusion protein expressed in Escherichia coli as a 34-kDa protein. Western blotting and RT-(real time) PCR analysis of DEV-infected cells showed that the protein was produced at the late stage of infection and that its production was highly dependent on viral DNA synthesis, suggesting that the gene should be classified as γ2 class. Analysis of extracellular virions revealed that the protein was a component of extracellular mature DEV virions. Indirect immunofluorescence studies localized most of the protein to the juxtanuclear region. These results will provide a basis for further functional analysis of the gene.






Similar content being viewed by others
References
Albrecht JC, Nicholas J, Biller D et al (1992) Primary structure of the herpesvirus saimiri genome. J Virol 66:5047–5058
An R, Li HX, Han ZX, Shao YH, Liu SW, Kong XG (2008) The ul31 to ul35 gene sequences of duck enteritis virus correspond to their homologs in herpes simplex virus 1. Acta Virol 52:23–30
Baer R, Bankier AT, Biggin MD et al (1984) DNA sequence and expression of the B95–8 Epstein-Barr virus genome. Nature 310:207–211
Burland TG (2000) DNASTAR’s lasergene sequence analysis software. Methods Mol Biol 132:71–91
Chang H, Cheng AC, Wang MS, Guo YF, Xie W, Lou KP (2009) Complete nucleotide sequence of the duck plague virus gE gene. Arch Virol 154:163–165
Chee MS, Bankier AT, Beck S et al (1990) Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol 154:125–169
Cheng AC, Wang MS, Wen M, Zhou WG, Guo YF, Jia RY, Xu C, Yuan GP, Liu YC (2006) Construction of duck enteritis virus gene libraries and discovery, cloning and identification of viral nucleocapsid protein gene. High Technol Lett 16:948–953
Clase AC, Lyman MG, del Rio T, Randall JA, Calton CM, Enquist LW, Banfield BW (2003) The pseudorabies virus Us2 protein, a virion tegument component, is prenylated in infected cells. J Virol 77:12285–12298
Daikoku T, Ikenoya K, Yamada H, Goshima F, Nishiyama Y (1998) Identification and characterization of the herpes simplex virus type 1 UL51 gene product. J Gen Virol 79:3027–3031
Davison AJ, Scott JE (1986) The complete DNA sequence of varicella-zoster virus. J Gen Virol 67:1759–1816
Elliott G, O’Hare P (2000) Cytoplasm-to-nucleus translocation of a herpesvirus tegument protein during cell division. J Virol 74:2131–2141
Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (2005) Virus taxonomy: classification and nomenclature of viruses: eighth report of the International Committee on the Taxonomy of Viruses. Academic Press, San Diego
Fuchs W, Ziemann K, Teifke JP, Werner O, Mettenleiter TC (2000) The non-essential UL50 gene of avian infectious laryngotracheitis virus encodes a functional dUTPase which is not a virulence factor. J Gen Virol 81:627–638
Gompels UA, Nicholas J, Lawrence G, Jones M, Thomson BJ, Martin ME, Efstathiou S, Craxton M, Macaulay HA (1995) The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology 209:29–51
Guo YF, Cheng AC, Wang MS, Zhou Y (2009) Purification of anatid herpesvirus 1 particles by tangential-flow ultrafiltration and sucrose gradient ultracentrifugation. J Virol Meth doi:10.1016/j.jviromet.2008.12.017
Hafezi W, Bernard E, Cook R, Elliott G (2005) Herpes simplex virus tegument protein VP22 contains an internal VP16 interaction domain and a C-terminal domain that are both required for VP22 assembly into the virus particle. J Virol 79:13082–13093
Hamel F, Boucher H, Simard C (2002) Transcriptional and translational expression kinetics of the bovine herpesvirus 1 UL51 homologue gene. Virus Res 84:125–134
Han XJ, Wang JW, Ma B (2006) Cloning and sequence of glycoprotein H gene of duck plague virus. Agric Sci China 5:397–402
Harms JS, Ren X, Oliveira SC, Splitter GA (2000) Distinctions between bovine herpesvirus 1 and herpes simplex virus type 1 VP22 tegument protein subcellular associations. J Virol 74:3301–3312
Higgins DG, Bleasby AJ, Fuchs R (1992) CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci 8:189–191
Homa FL, Brown JC (1997) Capsid assembly and DNA packaging in herpes simplex virus. Rev Med Virol 7:107–122
Hong-Yan Z, Murata T, Goshima F, Takakuwa H, Koshizuka T, Yamauchi Y, Nishiyama Y (2001) Identification and characterization of the UL24 gene product of herpes simplex virus type 2. Virus genes 22:321–327
Izumiya Y, Jang HK, Kashiwase H, Cai JS, Nishimura Y, Tsushima Y, Kato K, Miyazawa T, Kai C, Mikami T (1998) Identification and transcriptional analysis of the homologues of the herpes simplex virus type 1 UL41 to UL51 genes in the genome of nononcogenic Marek’s disease virus serotype 2. J Gen Virol 79:1997–2001
Jia RY, Cheng AC, Wang MS, Xin HY, Guo YF, Zhu DK, Qi XF, Zhao LC, Ge H, Chen X (2009) Analysis of synonymous codon usage in the UL24 gene of duck enteritis virus. Virus genes 38:96–103
Klupp BG, Granzow H, Klopfleisch R, Fuchs W, Kopp M, Lenk M, Mettenleiter TC (2005) Functional analysis of the pseudorabies virus UL51 protein. J Virol 79:3831–3840
Koshizuka T, Kawaguchi Y, Nozawa N, Mori I, Nishiyama Y (2007) Herpes simplex virus protein UL11 but not UL51 is associated with lipid rafts. Virus Genes 35:571–575
Lenk M, Visser N, Mettenleiter TC (1997) The pseudorabies virus UL51 gene product is a 30-kilodalton virion component. J Virol 71:5635–5638
Li BW, Rush AC, Tan J, Weil GJ (2004) Quantitative analysis of gender-regulated transcripts in the filarial nematode Brugia malayi by real-time RT-PCR. Mol Biochem Parasitol 137:329–337
Li HX, Liu SW, Kong XG (2006) Characterization of the genes encoding UL24, TK and gH proteins from duck enteritis virus (DEV): a proof for the classification of DEV. Virus genes 33:221–227
Liu FY, Ma B, Zhao Y, Zhang Y, Wu YH, Liu XM, Wang JW (2008) Characterization of the gene encoding glycoprotein C of duck enteritis virus. Virus Genes 37:328–332
Liu SW, Chen SH, Li HX, Kong XG (2007) Molecular characterization of the herpes simplex virus 1 (HSV-1) homologues, UL25 to UL30, in duck enteritis virus (DEV). Gene 401:88–96
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Loomis JS, Bowzard JB, Courtney RJ, Wills JW (2001) Intracellular trafficking of the UL11 tegument protein of herpes simplex virus type 1. J Virol 75:12209–12219
McGuire JM, Douglas M, Smith KD (1996) The resolution of the neutral N-linked oligosaccharides of IgG by high pH anion-exchange chromatography. Carbohydr Res 292:1–9
Mettenleiter TC (2002) Herpesvirus assembly and egress. J Virol 76:1537–1547
Miller WJ, Skinner JA, Foss GS, Davies KE (2000) Localization of the fragile X mental retardation 2 (FMR2) protein in mammalian brain. Eur J Neurosci 12:381–384
Mukhopadhyay A, Lee GE, Wilson DW (2006) The amino terminus of the herpes simplex virus 1 protein Vhs mediates membrane association and tegument incorporation. J Virol 80:10117–10127
Nozawa N, Daikoku T, Koshizuka T, Yamauchi Y, Yoshikawa T, Nishiyama Y (2003) Subcellular localization of herpes simplex virus type 1 UL51 protein and role of palmitoylation in Golgi apparatus targeting. J Virol 77:3204–3216
Nozawa N, Kawaguchi Y, Tanaka M, Kato A, Kato A, Kimura H, Nishiyama Y (2005) Herpes simplex virus type 1 UL51 protein is involved in maturation and egress of virus particles. J Virol 79:6947–6956
Pan H, Cao R, Liu L, Niu M, Zhou B, Chen P, Hu J (2008) Molecular cloning and sequence analysis of the duck enteritis virus UL5 gene. Virus Res 136:152–156
Plummer PJ, Alefantis T, Kaplan S, O’Connell P, Shawky S, Schat KA (1998) Detection of duck enteritis virus by polymerase chain reaction. Avian Dis 42:554–564
Qi XF, Yang XY, Cheng AC, Wang MS, Zhu DK, Jia RY (2008) Quantitative analysis of virulent duck enteritis virus loads in experimentally infected ducklings. Avian Dis 52:338–344
Sandhu TS, Shawky SA (2003) Duck virus enteritis. In: Saif YM (ed) Diseases of poultry, 11th edn. Wiley, New York, pp 354–363
Telford EA, Watson MS, McBride K, Davison AJ (1992) The DNA sequence of equine herpesvirus-1. Virology 189:304–316
Vittone V, Diefenbach E, Triffett D, Douglas MW, Cunningham AL, Diefenbach RJ (2005) Determination of interactions between tegument proteins of herpes simplex virus type 1. J Virol 79:9566–9571
Wada K, Goshima F, Takakuwa H, Yamada H, Daikoku T, Nishiyama Y (1999) Identification and characterization of the UL14 gene product of herpes simplex virus type 2. J Gen Virol 80:2423–2431
Wolf K, Burke CN, Quimby MC (1976) Duck viral enteritis: a comparison of replication by CCL-141 and primary cultures of duck embryo fibroblasts. Avian Dis 20:447–454
Xie W, Cheng AC, Wang MS, Chang H, Zhu DK, Luo QH, Jia RY, Chen XY (2009) Expression and characterization of the UL31 protein from Duck enteritis virus. Virol J 6:19
Yamauchi Y, Kiriyama K, Kubota N, Kimura H, Usukura J, Nishiyama Y (2008) The UL14 tegument protein of herpes simplex virus type 1 is required for efficient nuclear transport of the alpha transinducing factor VP16 and viral capsids. J Virol 82:1094–1106
Yuan GP, Cheng AC, Wang MS, Liu F, Han XY, Liao YH, Xu C (2005) Electron microscopic studies of the morphogenesis of duck enteritis virus. Avian Dis 49:50–55
Zhao LC, Cheng AC, Wang MS, Yuan GP, Jia RY, Zhou DC, Qi XF, Ge H, Sun T (2008) Identification and characterization of duck enteritis virus dUTPase gene. Avian Dis 52:324–331
Zhao Y, Wang JW, Liu F, Ma B (2009) Molecular analysis of US10, S3, and US2 in duck enteritis virus. Virus Genes doi:10.1007/s11262-008-0315-0
Zhao Y, Wang JW, Ma B, Liu F (2009) Molecular analysis of duck enteritis virus US3, US4, and US5 gene. Virus Genes 38:289–294
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (No. 30771598), Changjiang Scholars and Innovative Research Team in University (No. PCSIRT0848), the earmarked fund for Modern Agro-industry Technology Research System (No. nycytx-45-12), the Cultivation Fund of the Key Scientific and Technical Innovation Project, the Ministry of Education of China (No. 706050), the Cultivation Fund of the Key Scientific and Technical Innovation Project, Department of Education of Sichuan Province (No. 07ZZ028), the Sichuan Province Outstanding Youths Fund (No. 07ZQ026-132) and the Sichuan Province Basic Research Program (No. 07JY029-016/07JY029-017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Chan-Juan Shen, An-Chun Cheng, Ming-Shu Wang and Yu-Fei Guo were contributed equally to this work.
Rights and permissions
About this article
Cite this article
Shen, CJ., Cheng, AC., Wang, MS. et al. Identification and characterization of the duck enteritis virus UL51 gene. Arch Virol 154, 1061–1069 (2009). https://doi.org/10.1007/s00705-009-0407-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-009-0407-8